Abstract
Objectives
To determine how infant feeding recommendations can maximize HIV-free survival (HFS) among HIV-exposed, uninfected African infants, balancing risks of breastmilk-associated HIV infection with setting-specific risks of illness and death associated with replacement feeding.
Design
Validated mathematical model of HIV-exposed, uninfected infants, with published data from Africa.
Methods
We projected 24-month HFS using combinations of: 1) maternal CD4, 2) antiretroviral (ARV) availability, and 3) relative risk of mortality among replacement-fed compared to breastfed infants (“RR-RF,” range: 1.0–6.0). For each combination, we identified the “optimal” breastfeeding duration (0–24 months) maximizing HFS. We compared HFS under an “individualized” approach, based on the above parameters, to the World Health Organization (WHO) “public health approach” (12 months’ breastfeeding for all HIV-infected women).
Results
Projected HFS was 65–93%. When RR-RF=1.0, replacement feeding from birth maximized HFS. At a commonly reported RR-RF value (2.0), optimal breastfeeding duration was 3–12 months, depending on maternal CD4 and ARV availability. As RR-RF increased, optimal breastfeeding duration increased. Compared to the public health approach, an individualized approach improved absolute HFS by <1% if RR-RF=2.0–4.0, by 3% if RR-RF=1.0 or 6.0, and by greater amounts if access to ARVs was limited.
Conclusions
Tailoring breastfeeding duration to maternal CD4, ARV availability, and local replacement feeding safety can optimize HFS among HIV-exposed infants. An individualized approach leads to moderate gains in HFS, but only when mortality risks from replacement feeding are very low or very high, or ARV availability is limited. The WHO public health approach is beneficial in most resource-limited settings.
Keywords: HIV, mother-to-child transmission, PMTCT, infant feeding, breastfeeding
INTRODUCTION
Breastfeeding is critical to infant health and survival in many resource-limited settings [1]. Breastmilk provides optimal nutrition, especially where access to replacement milk is limited; passive transfer of maternal antibody protects against infectious diseases such as diarrhea and pneumonia; and exclusive breastfeeding avoids exposure to water contaminated with enteric pathogens [1–3]. For HIV-infected mothers, however, breastfeeding also places HIV-uninfected infants at risk of HIV infection. In the absence of antiretroviral drugs (ARVs) for prophylaxis, up to 40% of mother-to-child HIV transmission (MTCT) worldwide is attributed to breastfeeding [4, 5]. Maternal or infant ARVs can markedly reduce breastfeeding-related MTCT, but the risk of HIV transmission is not completely eliminated, and the residual risk remains greatest for women with advanced HIV disease (CD4 count ≤350/μL). [6–12].
The World Health Organization (WHO) 2010 and 2013 HIV and Infant Feeding Guidelines address this dilemma facing HIV-infected women and emphasize a goal of infant HIV-free survival (HFS) to simultaneously consider risks of HIV and those of infection-related death [1, 13]. To simplify infant feeding recommendations, the WHO promotes a “public health approach.” The guidelines recommend the choice at country- or program-level between avoidance of breastfeeding, suggested where water supplies are safe and infant formula quantity is adequate, and 12 months of breastfeeding with infant or maternal ARV prophylaxis (Appendix Table A) [1, 13]. However, infant HFS might be maximized by individualized recommendations regarding breastfeeding duration, in which risks of MTCT are balanced against risks of replacement feeding-associated mortality (Appendix Figure A). Such an individualized approach may be difficult to implement in maternal-child health settings. As neither clinical trial nor fully comprehensive cohort data exist to address this question, we used a validated computer simulation model to investigate the duration of breastfeeding that maximizes HFS for HIV-exposed, uninfected infants who live in settings of alternative replacement feeding-associated risk [14, 15].
METHODS
Analytic overview
We used the Cost-Effectiveness of Preventing AIDS Complications (CEPAC)-infant model to simulate HIV-exposed, uninfected infants in sub-Saharan Africa [14, 15]. The primary outcome was 24-month HFS, defined as being alive and HIV-uninfected at 24 months after birth. Although PMTCT regimens are chosen at the program or national level, water safety and the availability of ARVs and infant formula may vary, even between patients in a given program. To simulate conditions faced by individual women, we modeled numerous scenarios, reflecting variations in: 1) maternal CD4 distribution, 2) ARV availability, and 3) the relative risk (RR) of mortality associated with replacement feeding (RF) compared to breastfeeding (“RR-RF”), varied from 1.0 (no increased mortality risk associated with RF) to 6.0 (e.g., poor water quality, insufficient quantities of replacement milk, or diarrheal outbreaks) [5, 6, 16–21].
First, we projected infant 24-month HFS at breastfeeding durations of 0, 3, 6, 9, 12, 18, and 24 months, and identified the breastfeeding duration that maximized projected 24-month HFS for each combination of maternal disease stage, ARV availability, and RR-RF value. We termed this the “individualized approach,” because it might allow a provider to counsel a new mother regarding how long she should breastfeed, given information about her CD4 count, medication use, and the safety and adequacy of the water and replacement milk supply available to her. Second, we compared these results to the projected HFS with 12 months of breastfeeding by all women in the multinational MTCT-Plus cohort (the WHO “public health approach”) [1, 22].
Modeled population
We assigned HIV-exposed, uninfected infants born in sub-Saharan Africa to one of five categories of maternal CD4 and maternal/infant ARV availability: 1) maternal CD4 ≤350/μL, with no postnatal ARVs available, 2) maternal CD4 >350/μL, with no postnatal ARVs available, 3) maternal CD4 ≤350/μL, with lifelong maternal postnatal three-drug ART, 4) maternal CD4 >350/μL, with infant nevirapine throughout breastfeeding, and 5) maternal CD4 >350/μL, with postnatal maternal three-drug ARV prophylaxis throughout breastfeeding [13, 23, 24]. These categories reflected a range of MTCT risks under 2006, 2010, and 2013 WHO-recommended regimens for prevention of MTCT (PMTCT). Although 2013 WHO guidelines suggest a CD4 threshold of ≤500/μL for initiation of ART in non-pregnant patients, few data are available to model the WHO-recommended “Option A” and “Option B” PMTCT regimens using this threshold [2, 6, 10–13, 16, 25–33]. In addition, WHO 2013 guidelines recommend Option B or B+, in which all pregnant women initiate ART regardless of CD4 threshold [13]. Like other investigators, we therefore used a CD4 threshold of 350/μL to stratify risks of postnatal HIV transmission [34].
Model structure
CEPAC-infant model
The CEPAC-infant model is a first-order, Monte Carlo simulation model of infant HIV infection and survival, detailed in the Appendix [14, 15, 35]. For this analysis, infants entered the model at birth and faced monthly risks of three key events: 1) maternal death, after which infants were no longer at risk for HIV infection due to cessation of breastfeeding, but faced higher mortality rates due to maternal orphanhood [36–39]; 2) postnatal HIV infection throughout the duration of breastfeeding, stratified by maternal CD4 and maternal/infant ARV regimen [2, 6, 10–12, 16, 25–33]; and 3) all-cause infant mortality, stratified by age, infant HIV infection status, receipt of ART if infected, and maternal vital status. Baseline rates of infant mortality were applied to breastfed infants [8, 40]; for replacement-fed infants, these mortality rates were multiplied by published values of the “RR-RF” multiplier [5, 6, 16–21]. Model outcomes include true infant HIV and vital status, allowing calculation of cohort HIV-free survival at the end of each simulated month.
Breastfeeding type and duration
When modeled breastfeeding duration was 0 months, infants were assumed to initiate RF immediately after birth. For all other modeled breastfeeding durations (3–24 months), infants were assumed to wean after the last month of breastfeeding. In the base case, breastfeeding in the first 6 months of life was modeled as exclusive breastfeeding, per WHO guidelines [1, 13], and breastfeeding after 6 months of age was modeled as complementary feeding, combining breastmilk with other solid and liquid foods. We examined the impact of mixed breastfeeding in the first 6 months of life in sensitivity analyses.
Model input parameters
Postnatal transmission risks
Postnatal MTCT risks were derived from prevention of MTCT (PMTCT) studies among breastfed, African infants, stratified by maternal/infant ARV regimen and maternal CD4 count (Table 1, Section I) [2, 6, 10–12, 16, 25–33, 41]. We applied these risks from birth through cessation of breastfeeding [4]. In the base case, MTCT risks were the average of published values for each regimen and current maternal CD4 count. We then varied these using the highest and lowest published MTCT rates for each regimen simultaneously, as a proxy for maternal and infant ARV adherence throughout breastfeeding (“highest MTCT risk” and “lowest MTCT risk” scenarios; Table 1 and Appendix) [15]. Infants were assumed to experience no risk of HIV acquisition after breastfeeding stopped.
Table 1.
Selected model input parameters for the CEPAC-infant model
| I. Mother-to-child transmission risks in postnatal period (rate/100 person-years, applied to infants HIV-uninfected at birth)
| ||||
|---|---|---|---|---|
| Base-case value (range for sensitivity analyses) a | ||||
|
| ||||
| Maternal CD4 | Postnatal PMTCT regimen received | |||
| No ARVs | Extended infant NVP | Three-drug ART | Data sources | |
| ≤350/μL | 9.1 (EBF); 15.4 (MBF) (5.7–28.4) | n/a | 4.0 (0–6.4) | [2, 6, 10, 12, 25, 27, 31–33] |
| >350/μL | 2.9 (EBF); 4.8 (MBF) (1.8–8.8) | 2.7 (1.4–3.7) | 2.2 (0–6.4) | [2, 11, 12, 16, 25–30] |
| II. Monthly risk of infant mortality among breastfed, HIV-exposed, uninfected infants [8, 40]
| |
|---|---|
| Age (months) b | Base-case value (range for sensitivity analyses) |
| 0–2 | 0.0101 (0.0091–0.0115) |
| 3–5 | 0.0041 (0.0038–0.0045) |
| 6–11 | 0.0028 (0.0028–0.0030) |
| 12–17 | 0.0014 (0.0012–0.0014) |
| 18–23 | 0.0007 (0.0005–0.0009) |
| III. Maternal mortality
| ||
|---|---|---|
| Maternal HIV/ARV status | Monthly risk | Data source |
| CD4≤350/μL, no ARVs | 0.0078 | Projected from CEPAC-International adult model (see Supplemental Appendix) |
| CD4>350/μL, no ARVs | 0.0024 | |
| CD4≤350/μL, maternal ART | 0.0016 | |
| CD4>350/μL, maternal ART | 0.0009 | |
| IV. Relative risk of mortality among replacement-fed compared to breastfed infants (RR-RF) c
| ||
|---|---|---|
| Reported values | Setting | References |
| 1.0 | Kenya; Rwanda; South Africa; Côte d’lvoire | [5, 16–18] |
| 2.0 | Botswana | [6] |
| 1.8–3.3 | Malawi | [19] |
| 2.0–4.2 | Zambia | [20, 42] |
| 6.0 | Uganda | [21] |
PMTCT: prevention of mother-to-child HIV transmission; EBF: exclusive breastfeeding (in first six months of life, followed by complementary feeding thereafter); MBF: mixed breastfeeding (in first six months of life, followed by complementary feeding thereafter); m: months; ARVs: antiretroviral drugs; ART: three-drug antiretroviral therapy.
The ranges shown for sensitivity analyses are the highest and lowest published values for each regimen. For the “highest MTCT risk” scenario, we used the upper limit of each range; for the “lowest MTCT risk” scenario, we used the lower limit. These MTCT ranges may represent a proxy for adherence to maternal and infant ARVs in PMTCT trials.
Mortality rates are stratified by current age in each month of the simulation.
The RR-RF was calculated by dividing cumulative mortality risk (at the greatest duration reported in each study) among replacement-fed infants by cumulative mortality risk among breastfed infants.
Mortality rates
Infant mortality rates were from UNAIDS analyses of pooled data for HIV-exposed, uninfected breastfeeding infants in eight African countries, stratified by age (Table 1, Section II) [8, 40]. In sensitivity analyses, we examined “low-mortality” (UNAIDS lower 95% confidence limit) and “high-mortality” (UNAIDS upper 95% confidence limit) scenarios. Rates of maternal mortality were projected using the CEPAC-adult model (Table I, Section III; Appendix).
RR-RF values and duration
We derived values for the RR-RF mortality multiplier from published studies reporting all-cause mortality among HIV-exposed, breastfed and replacement-fed infants (Table 1, Section IV) [5, 6, 16–21]. In the base-case analysis, RR-RF was modeled to apply from initiation of RF until the end of the simulation (month 24). It is unknown whether the highest risk of mortality from shorter breastfeeding occurs at specific high-vulnerability ages, or instead for a high-risk period immediately after weaning [3, 6, 20, 42, 43]. We therefore conducted two sensitivity analyses. First, we applied the RR-RF mortality multiplier only until infants reached specific ages (i.e., until 6 or 12 months of age, for infants weaned prior to those ages). Second, we applied the RR-RF mortality multiplier only for specific durations after weaning (i.e., for 1, 3, 6, or 12 months after weaning, regardless of the age at weaning).
Model validation
The CEPAC-infant model has previously been validated against published risks of postnatal infant HIV infection and mortality [14]. We further validated the model for the outcome of HFS, using a published comparison of breastfeeding plus maternal ART vs. replacement feeding in Rwanda (Appendix) [16].
Additional sensitivity analyses
Following international modeling recommendations, we conducted extensive univariate and multivariate sensitivity analyses on all key model input parameters, through ranges shown in Table 1 [44, 45]. In addition to the sensitivity analyses outlined for the parameters above, we also varied the impact of maternal mortality on infant mortality (range, 1–2-fold increase in infant mortality risk following maternal death) [36] and the proportion of infants who were mixed breastfed in the first six months of life (range, 0–100%).
Breastfeeding recommendations: individualized vs. public health approach
The WHO 2010 HIV and Infant Feeding Guidelines include 16 principles and recommendations, encompassing a range of maternal and infant health-related interventions (Appendix Table A). Although Principles 5 and 6 include counseling, they deliberately focus on “supporting mothers in their feeding practices” rather than “a process of decision making.” Most programs have emphasized Principle 3 and Recommendation 2, which suggest 12 months of breastfeeding with maternal or infant ARV prophylaxis for all HIV-infected women. We termed this the “public health approach.” To compare the individualized and public health approaches, we simulated a cohort of women and infants similar to the multi-country MTCT-Plus cohort, in which 2,741 of 6,036 women (45%) had CD4 ≤350/μL [22]. We projected 24-month HFS, assuming Option B/B+ (ART throughout breastfeeding; Appendix Table A) [1]. To reflect a range of settings, we varied both RR-RF (from 1.0–6.0) and the “uptake” of recommended ARVs (proportion of mother/infant pairs receiving and adhering to recommended regimens; range, 50–100%). Because the individualized approach may be burdensome to healthcare providers, we modeled two additional scenarios in which providers were unable to give any infant feeding counseling to 25% of mothers under the individualized strategy, for example, leading them to breastfeed for 24 months without ARV prophylaxis or to replacement feed from birth (Appendix) [1, 46].
RESULTS
Model validation
Model-projected HFS was within 2% of observed HFS for both replacement-fed and breastfed infants, at 30 to 270 days after birth (Appendix Table B) [16].
Base-case results (Table 2; Figure 1)
Table 2.
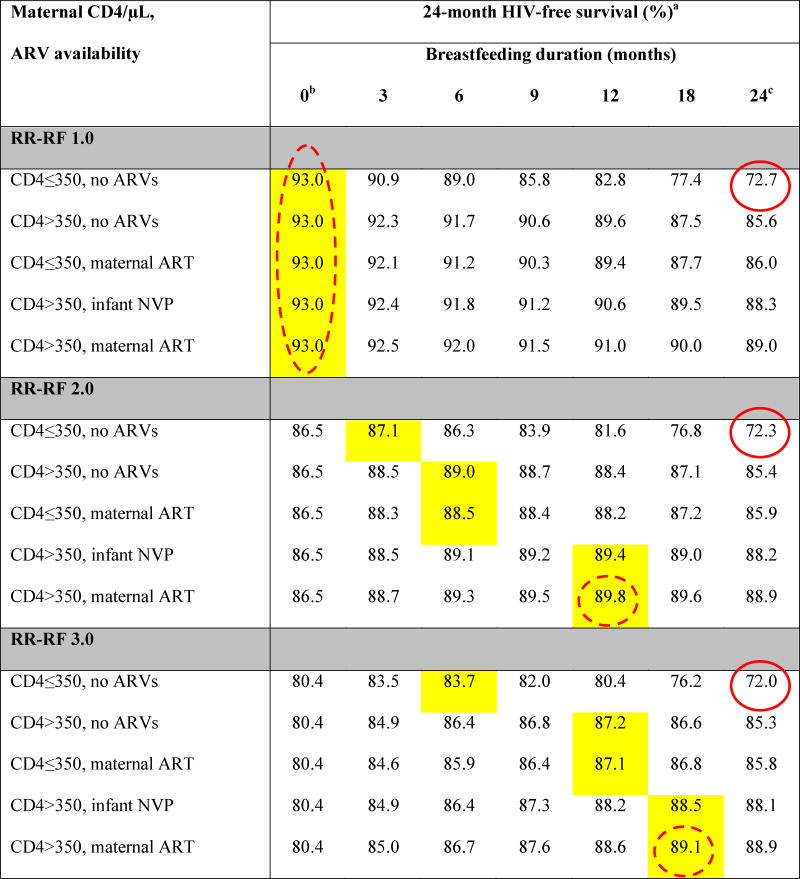
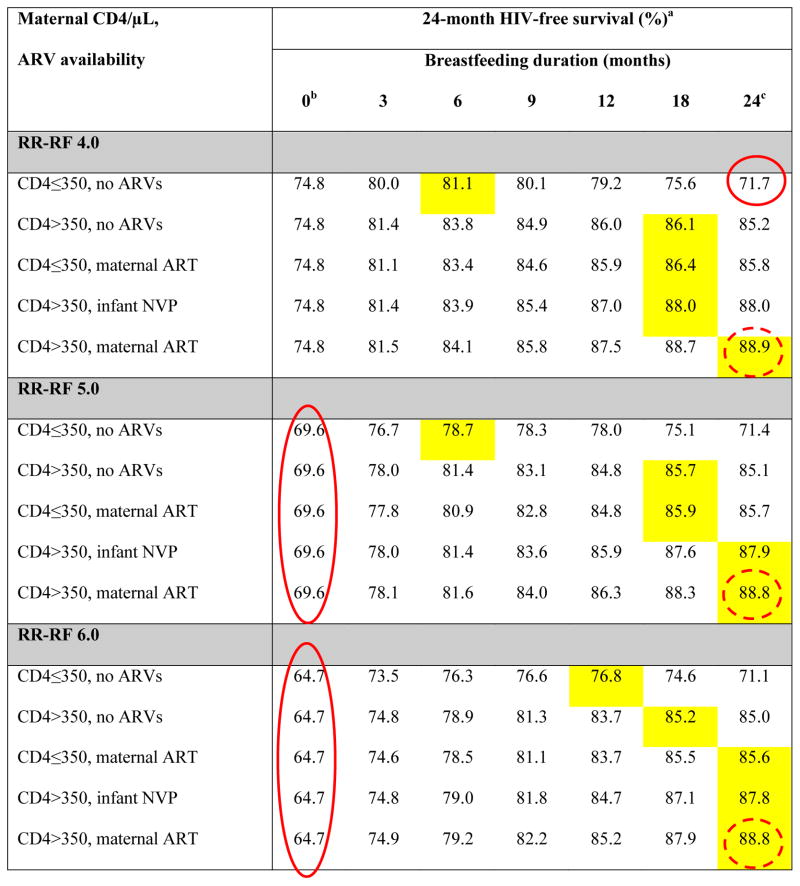
Base-case results: Projected 24-month HIV-free survival among HIV-exposed, uninfected infants at birth
|
|
ARVs: antiretroviral drugs; ART: 3-drug antiretroviral therapy; NVP: nevirapine; RR-RF: relative risk of mortality among replacement-fed compared to breastfed infants (see Methods).
Yellow shading indicates the maximum value of HIV-free survival (HFS) for each unique combination of RR-RF value, maternal CD4 count and ARV availability. Circled values are the high HFS (solid circles) and low HFS (dashed circles) for each value of RR-RF.
When breastfeeding duration is 0 months, HFS is equivalent for all CD4/ARV categories for each value of RR-RF. Because we assume no MTCT risk with replacement feeding from birth, HFS is determined solely by mortality, which depends on RR-RF, but not on maternal CD4 or ARV availability.
In all scenarios, the RR-RF is not applied to infants who are breastfed for 24 months (weaning occurs at the end of the simulation). For each combination of maternal CD4 and ARV availability, HFS at breastfeeding durations of 24 months decreases slightly as the RR-RF increases (for example, with CD4>350 and maternal ART, HFS decreases from 89.0 at RR-RF=1.0 to 88.8 at RR-RF=6.0). This occurs because infants necessarily wean if maternal mortality occurs, and the RR-RF then takes effect for the small proportion of infants whose mothers have died.
Figure 1. Base-case results: optimal breastfeeding duration to maximize infant 24-month HIV-free survival.
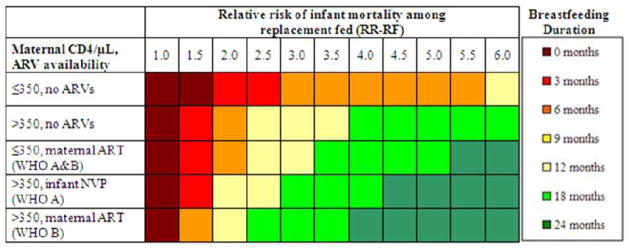
For each combination of maternal CD4, ARV availability, and RR-RF, colored shading indicates the breastfeeding duration maximizing 24-month infant HIV-free survival.. RR-RF: relative risk of mortality among replacement-fed compared to breastfed infants; ARVs: antiretroviral drugs; ART: three-drug antiretroviral therapy; NVP: nevirapine; WHO A and B: World Health Organization-recommended PMTCT regimens.
For all scenarios, projected HFS ranged from a low of 64.7% (RR-RF=6.0, no breastfeeding, solid circle in Table 2) to a high of 93.0% (RR-RF=1.0, no breastfeeding; dashed circle in Table 2). Overall, HFS was inversely related to RR-RF and directly related to maternal CD4 and ARV availability.
The RR-RF value of 1.0 represented a unique case (Figure 1, Table 2): HFS was maximized (93.0%) by RF from birth for all combinations of maternal CD4 and ARV availability. At RR-RF=1.0, any duration of breastfeeding led only to increased MTCT risk, without reduction in RF-associated mortality. HFS therefore declined steadily with increasing breastfeeding duration, to nadir values ranging from 72.7% (maternal CD4 ≤350/μL, no ARVs, solid circle in Table 2) to 89.0% (maternal CD4 >350/μL, maternal ART).
For RR-RF values >1.0, the breastfeeding duration that maximized projected HFS depended on the interaction of maternal CD4, ARV availability, and the RR-RF (Table 2, Figure 1). For example, at the commonly reported RR-RF of 2.0, HFS ranged from 72.3% (Table 2, solid circle) - 89.8% (dashed circle) [6, 19, 20, 42]. In the scenario with highest MTCT risk (maternal CD4 ≤350/μL and no ARVs) at RR-RF=2.0, infant HFS was maximized with 3 months of breastfeeding (87.1%); beyond 3 months of breastfeeding, the risk of HIV infection outweighed the risk of mortality associated with replacement feeding. In maternal CD4/ARV scenarios with lower MTCT risks, longer breastfeeding durations were preferred (Table 2). As RR-RF increased beyond 2.0, the mortality reductions associated with breastfeeding became relatively greater than the risks of MTCT, and HFS was maximized with longer breastfeeding durations. At the extreme RR-RF value of 6.0, HFS ranged from 64.7% (solid circle) to 88.8% (dashed circle) and was maximized by breastfeeding durations of 12 months (maternal CD4 ≤350/μL, no ARVs; HFS, 76.8%), 18 months (CD4 >350/μL, no ARVs; HFS, 85.2%), and 24 months (all other CD4/ARV categories; HFS 85.6–88.8%; Table 2; Figure 1).
Sensitivity analyses (Table 3, Appendix Tables C-F)
Table 3.
Selected sensitivity analyses: optimal breastfeeding duration to maximize HIV-free survival among HIV-exposed, uninfected infants at birth
| Maternal CD4/μL, ARV availability | Base case | Lowest MTCT risks | Highest MTCT risks | RR-RF applies until age 6m | RR-RF applies until age 12m | RR-RF applies for 1m after wean | RR-RF applies for 3m after wean | RR-RF applies for 6m after wean | RR-RF applies for 12m after wean | Mixed feeding in first 6m of life |
|---|---|---|---|---|---|---|---|---|---|---|
| Duration of breastfeeding (months) at which 24-month HIV-free survival is maximizeda,b | ||||||||||
|
| ||||||||||
| RR-RF 2.0 | ||||||||||
|
| ||||||||||
| CD4≤350, no ARVs | 3 | 3 | 0 | 3 | 3 | 0 | 0 | 0 | 3 | 0 |
| CD4>350, no ARVs | 6 | 12 | 3 | 6 | 6 | 3 | 3 | 3 | 6 | 6 |
| CD4≤350, maternal ART | 6 | 24 | 3 | 6 | 6 | 0 | 3 | 3 | 3 | 6 |
| CD4>350, infant NVP | 12 | 18 | 6 | 6 | 12 | 3 | 3 | 3 | 6 | 12 |
| CD4>350, maternal ART | 12 | 24 | 3 | 6 | 12 | 3 | 3 | 6 | 12 | 12 |
|
| ||||||||||
| RR-RF 3.0 | ||||||||||
|
| ||||||||||
| CD4≤350, no ARVs | 6 | 12 | 3 | 6 | 6 | 0 | 3 | 3 | 3 | 3 |
| CD4>350, no ARVs | 12 | 24 | 6 | 6 | 12 | 3 | 6 | 6 | 12 | 12 |
| CD4≤350, maternal ART | 12 | 24 | 9 | 6 | 12 | 3 | 3 | 3 | 12 | 12 |
| CD4>350, infant NVP | 18 | 24 | 12 | 6 | 12 | 3 | 6 | 12 | 18 | 18 |
| CD4>350, maternal ART | 18 | 24 | 12 | 6 | 12 | 3 | 6 | 12 | 18 | 18 |
|
| ||||||||||
| RR-RF 4.0 | ||||||||||
|
| ||||||||||
| CD4≤350, no ARVs | 6 | 12 | 3 | 6 | 6 | 0 | 3 | 3 | 6 | 3 |
| CD4>350, no ARVs | 18 | 24 | 12 | 6 | 12 | 3 | 6 | 12 | 18 | 18 |
| CD4≤350, maternal ART | 18 | 24 | 12 | 6 | 12 | 3 | 6 | 12 | 18 | 18 |
| CD4>350, infant NVP | 18 | 24 | 18 | 6 | 12 | 3 | 6 | 12 | 18 | 18 |
| CD4>350, maternal ART | 24 | 24 | 12 | 6 | 12 | 3 | 12 | 24 | 18 | 24 |
|
| ||||||||||
| RR-RF 5.0 | ||||||||||
|
| ||||||||||
| CD4≤350, no ARVs | 6 | 18 | 6 | 6 | 6 | 0 | 3 | 3 | 6 | 6 |
| CD4>350, no ARVs | 18 | 24 | 12 | 6 | 12 | 3 | 6 | 12 | 18 | 18 |
| CD4≤350, maternal ART | 18 | 24 | 18 | 6 | 12 | 3 | 6 | 12 | 18 | 18 |
| CD4>350, infant NVP | 24 | 24 | 18 | 6 | 12 | 3 | 12 | 24 | 24 | 24 |
| CD4>350, maternal ART | 24 | 24 | 18 | 6 | 12 | 6 | 12 | 24 | 24 | 24 |
|
| ||||||||||
| RR-RF 6.0 | ||||||||||
|
| ||||||||||
| CD4≤350, no ARVs | 12 | 18 | 6 | 6 | 12 | 3 | 3 | 3 | 6 | 12 |
| CD4>350, no ARVs | 18 | 24 | 12 | 6 | 12 | 3 | 6 | 12 | 18 | 18 |
| CD4≤350, maternal ART | 24 | 24 | 18 | 6 | 12 | 3 | 12 | 24 | 24 | 24 |
| CD4>350, infant NVP | 24 | 24 | 24 | 6 | 12 | 3 | 12 | 24 | 24 | 24 |
| CD4>350, maternal ART | 24 | 24 | 18 | 6 | 12 | 6 | 12 | 24 | 24 | 24 |
MTCT: mother-to-child HIV transmission; RR-RF: relative risk of mortality among replacement-fed compared to breastfed infants: m: months; ARVs: antiretroviral drugs; ART: 3-drug antiretroviral therapy; NVP: nevirapine.
Changes in policy conclusions from the base case are indicated by shading: dark orange indicates an optimal breastfeeding duration longer than in the base case, light orange indicates an optimal breastfeeding duration shorter than in the base case, and no shading indicates no change from the base case.
Projected HIV-free survival and results of additional sensitivity analyses are in Appendix Tables C-F, or available from the authors upon request. Results for RR-RF of 1.0 are not shown here, as the optimal breastfeeding duration remained 0 months in all sensitivity analyses.
Results of univariate and multivariate sensitivity analyses that led to changes in optimal breastfeeding duration are shown in Table 3 and Appendix Tables C-F. The “lowest MTCT risk” scenario, likely reflecting optimal adherence to maternal or infant ARVs, led to longer optimal breastfeeding durations than the base-case analysis, up to 24 months of breastfeeding in most scenarios when RR-RF was at least 3.0 (Appendix Table C). In contrast, most “highest MTCT risk” scenarios, likely reflecting poorer adherence, favored shorter breastfeeding durations than the base case (Appendix Table D). When the RR-RF was applied for more limited time periods than in the base case, HFS was maximized by weaning at earlier ages (Appendix Tables E-F). However, if the RR-RF applied for 12 months after weaning, optimal breastfeeding durations were very similar to the base-case results. In addition to these analyses, we also varied all other key model inputs; the optimal breastfeeding duration was relatively insensitive to variations in the proportion of infants mixed breastfed in the first six months of life, infant mortality rates, or increased risk of infant mortality following maternal mortality (not shown).
Individualized vs. public health approach (Figure 2, Supplemental Table G)
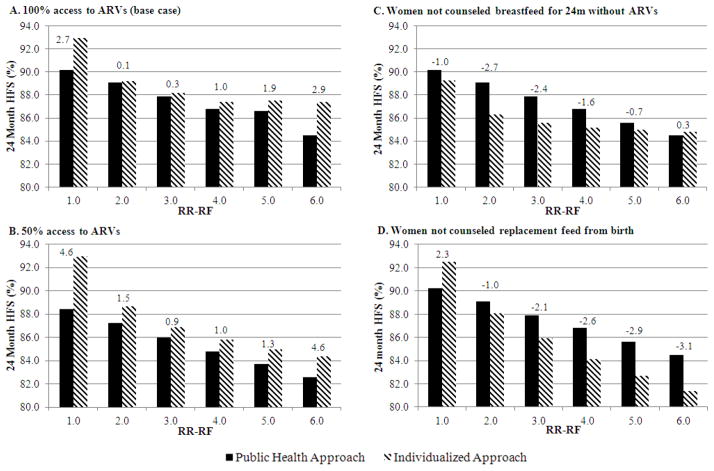
Figure 2. 24-month HIV-free survival with individualized approach, compared to “public health approach” recommending 12 months of breastfeeding for all HIV-infected women.
In each panel, the horizontal axis includes six values of RR-RF, ranging from 1.0 to 6.0. The vertical axis shows 24-month HIV-free survival, with shaded bars representing projections for the individualized approach and solid bars representing projections for the public health approach. Panels A and B depict scenarios in which providers counsel all HIV-infected mothers, with varying access to maternal ARVs. Panels C and D depict scenarios in which the individualized approach diverts provider resources and 25% of mothers receive no counseling, leading them to breastfeed for 24 months without ARVs (Panel C) or replacement feed from birth (Panel D). The difference in HFS between the individualized and public health approaches is shown for each RR-RF value; positive values indicate greater HFS with the individualized approach. HFS: HIV-free survival; RR-RF: relative risk of mortality among replacement-fed compared to breastfed infants; ARVs: antiretroviral drugs; m: months.
For the MTCT-Plus cohort [22], assuming guideline-concordant care with Options B/B+, projected gains in 24-month infant HFS with the individualized approach compared to the public health approach were only 0.1% if RR-RF=2.0, and 0.3% if RR-RF=3.0 (Figure 2A). Gains in HFS increased to 1.0–1.9% if RR-RF was 4.0–5.0, and were greatest at the extreme values of RR-RF (2.7% if RR-RF=1.0, 2.9% if RR-RF=6.0). If ARVs were only available for 50% of mother/infant pairs (Figure 2B), gains in HFS with the individualized approach were greatest at the RR-RF value of 1.0 (4.6%), but smaller at RR-RF values of 2.0–6.0 (0.9–1.9%). If the individualized approach rendered healthcare workers unable to provide counseling to 25% of new mothers, leading them to either breastfeed for 24 months without ARVs or to avoid breastfeeding, the individualized approach reduced cohort HFS for select combinations of maternal CD4, ARV availability, and RR-RF (Figure 2C–D).
DISCUSSION
This model-based analysis has four key findings. First, HFS for HIV-exposed, uninfected infants is maximized by shorter breastfeeding durations if maternal HIV disease is advanced or postnatal ARVs are unavailable and by longer breastfeeding durations in settings where replacement feeding is associated with high infant/child mortality risks.
Second, the optimal breastfeeding duration depends substantially on postnatal MTCT risks for each combination of maternal CD4 and ARV regimen, which are primarily related to ARV adherence. Although low adherence to maternal antenatal ARV prophylaxis and postnatal ART has been reported, there are limited data about adherence to maternal or infant ARV prophylaxis (Options A and B) throughout the long durations required by extended breastfeeding [47–49]. Our base case incorporated average transmission risks observed in PMTCT trials, and we assumed constant transmission risks and adherence throughout breastfeeding [4]. Postnatal adherence may be substantially lower in non-trial settings, and adherence to maternal and infant ARVs may wane over time. These conditions are at least partly reflected in our “highest MTCT risk” scenarios, in which optimal breastfeeding durations are often shorter than the WHO 12-month recommendation, especially if RR-RF is low (Table 3). Conversely, if the lowest published MTCT risks are assumed, likely reflecting outstanding adherence to ARVs or longer durations of maternal ART prior to delivery [11, 12], the optimal breastfeeding duration approaches 24 months in most scenarios.
Third, the interaction between postnatal MTCT risk and RR-RF is important. Increases in RR-RF values exert less influence on optimal breastfeeding duration when ARVs are not available than when ARVs are available. However, even at moderate RR-RF values, HFS is greater when women breastfeed without ARVs than when breastfeeding is avoided altogether, especially for women with CD4 >350/μL. In most settings with RR-RF >1.0, women should not avoid breastfeeding while awaiting access to ARVs.
Fourth, these results suggest the possibility of tailoring infant feeding recommendations for individual patients. There are clear challenges to such an “individualized” approach. First, information about water safety and replacement milk supply in the community may not accurately predict the RR-RF for individual infants. Second, an individualized approach may be too cumbersome for healthcare workers to implement, and previous efforts to individualize infant feeding recommendations have led to confusion about feeding recommendations for both HIV-infected and HIV-uninfected women [1, 46, 50–52]. Our results suggest that the individualized approach could lead to moderate benefits compared to the public health approach, but only when RR-RF is very low or very high or ARV availability is limited; some providers may already be individualizing feeding recommendation in such situations [50, 52]. The individualized approach is likely of minimal benefit at the intermediate values of RR-RF expected in most settings, or as programs scale up the availability of maternal ART under Options B/B+. While model-based analyses cannot capture all of the challenges of individualization, we find that if the time required to individually counsel some mothers leads other mothers to receive no counseling at all, the individualized approach may actually reduce infant HFS (Figure 2C–D).
This work confirms and extends the results of prior model-based analyses, which reflected MTCT risk in the absence of maternal/infant ARV prophylaxis and identified either 6 months of breastfeeding or replacement feeding from birth as the optimal strategy in most settings [53–56]. Our analysis additionally incorporates: 1) use of postnatal maternal/infant ARVs; 2) transmission risks stratified by maternal HIV disease stage; 3) breastfeeding durations from 0–24 months (including the WHO-recommended 12-month duration); and 4) explicit variations in RR-RF to reflect a range of settings.
Model-based analyses necessarily simplify complex biologic and operational processes, and it is important to understand key limitations and their impact on model results. Several limitations of our study relate to data regarding mortality rates and RR-RF values, which combine many causes of infant mortality into a single parameter. First, we were unable to identify reports from which we could derive RR-RF values only for HIV-exposed, uninfected children, to whom it is applied in the CEPAC model. However, the inclusion of children who become HIV-infected, despite varying MTCT and HIV-related mortality rates, underestimates the protective effect of breastfeeding in each study. Second, we apply the same value of RR-RF for infants of all ages. One WHO report suggests that RR-RF may decline from ages 0–12 months then remain constant from ages 12–23 months, but this analysis excluded HIV-exposed children and African children [43]. Data from Zambia suggest increasing RR-RF values with older age at weaning, while other studies have suggested a “high-risk” period immediately after weaning at any age or following abrupt weaning [3, 6, 20, 42]. We therefore modeled RR-RF without stratification by age or weaning duration, but varied it explicitly through wide ranges (1.0–6.0), and we examined the impact of the ages and duration after weaning at which the RR-RF applied. Finally, we used UNAIDS-derived child mortality rates, reported from studies with excellent access to pediatric healthcare [40]. In sensitivity analyses, higher mortality rates that may be more generalizable to non-trial settings did not substantially change optimal breastfeeding durations.
Additional limitations relate to available data about MTCT risks. Our analysis excluded the CD4-independent impact of duration of antenatal ARV prophylaxis on postnatal MTCT risk. Women who initiate ART late in pregnancy or postpartum may not fully suppress HIV RNA in plasma and breastmilk by delivery, permitting early breastfeeding MTCT [1, 11]. We also excluded situations in which women continue breastfeeding after ARV prophylaxis is stopped, either intentionally or due to medication stockouts, or stop and then resume breastfeeding (re-lactation), thereby increasing HIV concentration in breastmilk [57]. True postnatal risks may approach our “lowest MTCT risk” scenario if maternal ART is initiated early in pregnancy and if women are supported to adhere to ARV and continuous breastfeeding, and may approach our “highest MTCT risk” scenario under opposite conditions.
We focused this model-based analysis on the outcome of HIV-free survival, and thus did not examine other potential impacts of the individualized and public health approaches on maternal-child health services for HIV-infected patients and the general population [46, 50, 51]. Notably, the WHO-recommended outcome of HFS reflects an implicit valuation of infant HIV infection as “equal” to death [1]. As access to early infant HIV diagnosis and pediatric ART improves, an endpoint of total 2-year survival may better reflect public health goals [58]. Such an endpoint would also incorporate the benefits of breastfeeding for infants already HIV-infected [59]. Analyses that examine strategies to maximize total 2-year survival and incorporate HIV-infected infants [56] will favor longer optimal breastfeeding durations than the current analysis.
Conclusions
Over 765 million people live without access to safe drinking water, including 37% of people living in sub-Saharan Africa [60, 61]. Additionally, only 65% of HIV-infected, pregnant women receive antiretroviral therapy in sub-Saharan Africa; a smaller percentage receive treatment in South and SouthEast Asia, the Middle East, and North Africa, and access to therapy during breastfeeding is likely lower still [62]. This confluence of public health challenges underscores the importance of balancing the health risks from unsafe drinking water with those of mother-to-child HIV transmission. By using recent data on MTCT risks with modern infant and maternal ARV regimens, and by comparing a range of mortality risks from lack of breastfeeding, this analysis provides an updated assessment of the WHO HIV and Infant Feeding Guidelines. Consideration of maternal CD4 count, ARV availability, and local/personal conditions affecting the safety of replacement feeding may allow providers to tailor infant feeding recommendations under very specific circumstances. Compared to current WHO recommendations [1], this individualized approach is projected to lead to improved infant HIV-free survival only in settings where the mortality risks associated with replacement feeding are very low or very high, or where access to ARVs for prophylaxis of postnatal HIV transmission remains limited. Our results suggest that the current WHO public health policy is beneficial in most resource-limited settings.
Supplementary Material
Acknowledgments
Dr. Ciaranello designed the analysis, conducted model analyses and interpreted model results, and drafted and revised the paper. Ms. Rusibamayila and Ms. Kelly contributed to the design of the analysis, conducted model analyses, interpreted model results, and drafted and revised the paper. Drs. Leroy, Freedberg, Shapiro, Engelsmann, Lockman, Dabis, and Walensky contributed to the design of the analysis, interpreted model results, and critically revised the paper. The authors gratefully acknowledge Sarah Christensen and Jordan Francke for assistance with manuscript preparation. We also thank the CEPAC-International and CEPAC-Pediatric research teams for their contributions.
Funding for this work was provided by the March of Dimes Foundation, the Massachusetts General Hospital Executive Committee on Research, and the National Institutes of Health, including the National Institute of Allergy and Infectious Disease (NIAID) through K01 AI078754 (ALC), K24 AI062476 (KAF), R01 AI058736 (KAF, AR, RPW), R01 AI093269 (RW), and the Center for AIDS Research/P30 AI060354 (KAF, SL, RPW), and the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT; KAF, AR, RPW). Overall support for IMPAACT was provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Support of the sites was provided by the National Institute of Allergy and Infectious Diseases (NIAID) and the NICHD International and Domestic Pediatric and Maternal HIV Clinical Trials Network funded by NICHD (contract number N01-DK-9-001/HHSN267200800001C).
Footnotes
Presentation of results: This work was presented in part at the Conference on Retroviruses and Opportunistic Infections (Poster #1008), Seattle, Washington, USA; March, 2012.
Financial Disclosure: All authors have no financial relationships relevant to this article to disclose.
Conflicts of interest: All authors have no conflicts of interest to disclose.
Ethics: This work was approved by the Partners Healthcare Human Subjects Committee, Boston, MA, USA.
References
- 1.World Health Organization. [Accessed February 25, 2014];Guidelines on HIV and infant feeding: Principles and recommendations for infant feeding in the context of HIV and a summary of evidence. 2010 at http://whqlibdoc.who.int/publications/2010/9789241599535_eng.pdf. [PubMed]
- 2.Kuhn L, Aldrovandi GM, Sinkala M, Kankasa C, Mwiya M, Thea DM. Potential impact of new WHO criteria for antiretroviral treatment for prevention of mother-to- child HIV transmission. AIDS. 2010;24:1374–1377. [PMC free article] [PubMed] [Google Scholar]
- 3.Shapiro RL, Lockman S. Mortality among HIV-exposed infants: the first and final frontier. Clin Infect Dis. 2010;50:445–447. doi: 10.1086/649887. [DOI] [PubMed] [Google Scholar]
- 4.Coutsoudis A, Dabis F, Fawzi W, Gaillard P, Haverkamp G, Harris DR, et al. Late postnatal transmission of HIV-1 in breast-fed children: an individual patient data meta-analysis. J Infect Dis. 2004;189:2154–2166. doi: 10.1086/420834. [DOI] [PubMed] [Google Scholar]
- 5.Nduati R, John G, Mbori-Ngacha D, Richardson B, Overbaugh J, Mwatha A, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA. 2000;283:1167–1174. doi: 10.1001/jama.283.9.1167. [DOI] [PubMed] [Google Scholar]
- 6.Thior I, Lockman S, Smeaton LM, Shapiro RL, Wester C, Heymann SJ, et al. Breastfeeding plus infant zidovudine prophylaxis for 6 months vs formula feeding plus infant zidovudine for 1 month to reduce mother-to-child HIV transmission in Botswana: a randomized trial: the Mashi Study. JAMA. 2006;296:794–805. doi: 10.1001/jama.296.7.794. [DOI] [PubMed] [Google Scholar]
- 7.Dabis F, Bequet L, Ekouevi DK, Viho I, Rouet F, Horo A, et al. Field efficacy of zidovudine, lamivudine and single-dose nevirapine to prevent peripartum HIV transmission. AIDS. 2005;19:309–318. [PMC free article] [PubMed] [Google Scholar]
- 8.Marston M, Becquet R, Zaba B, Moulton LH, Gray G, Coovadia H, et al. Net survival of perinatally and postnatally HIV-infected children: a pooled analysis of individual data from sub-Saharan Africa. Int J Epidemiol. 2011;40:385–396. doi: 10.1093/ije/dyq255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marazzi MC, Liotta G, Haswell J, Zimba I, Nielsen-Saines K, Maulidi M, et al. TUAC101: Extended use of highly active antiretroviral therapy (HAART) during pregnancy in Southern Africa is highly protective in HIV-1 prevention of mother-to-child-transmission (PMTCT) also in women with higher CD4 cell counts. 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention; Cape Town, South Africa. 2009. [Accessed February 15, 2014]. at http://www.iasociety.org/Abstracts/A200721958.aspx. [Google Scholar]
- 10.Kesho Bora Study Group. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis. 2011;1:159. doi: 10.1016/S1473-3099(10)70288-7. [DOI] [PubMed] [Google Scholar]
- 11.Chasela CS, Hudgens MG, Jamieson DJ, Kayira D, Hosseinipour MC, Kourtis AP, et al. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med. 2010;362:2271–2281. doi: 10.1056/NEJMoa0911486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shapiro RL, Hughes MD, Ogwu A, Kitch D, Lockman S, Moffat C, et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med. 2010;362:2282–2294. doi: 10.1056/NEJMoa0907736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. [Accessed February 6, 2014];Consolidated guidelines on the use of antiretrovirals for the treatment and prevention of HIV infection. 2013 at http://www.who.int/hiv/pub/guidelines/arv2013/download/en/index.html.
- 14.Ciaranello AL, Perez F, Maruva M, Chu J, Englesmann B, Keatinge J, et al. WHO 2010 guidelines for prevention of mother-to-child HIV transmission in Zimbabwe: Modeling clinical outcomes in infants and mothers. PLoS ONE. 2011;6:e20224. doi: 10.1371/journal.pone.0020224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciaranello A, Perez F, Keatinge J, Park J, Engelsmann B, Maruva M, et al. What will it take to eliminate pediatric HIV? Reaching “virtual elimination” targets for prevention of mother-to-child HIV transmission (PMTCT) in Zimbabwe. PLoS Medicine. 2012;9:e1001156. doi: 10.1371/journal.pmed.1001156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peltier CA, Ndayisaba GF, Lepage P, van Griensven J, Leroy V, Pharm CO, et al. Breastfeeding with maternal antiretroviral therapy or formula feeding to prevent HIV postnatal mother-to-child transmission in Rwanda. AIDS. 2009;23:2415–2423. doi: 10.1097/QAD.0b013e32832ec20d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rollins NC, Becquet R, Bland RM, Coutsoudis A, Coovadia HM, Newell ML. Infant feeding, HIV transmission and mortality at 18 months: the need for appropriate choices by mothers and prioritization within programmes. AIDS. 2008;22:2349–2357. doi: 10.1097/QAD.0b013e328312c740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becquet R, Bequet L, Ekouevi DK, Viho I, Sakarovitch C, Fassinou P, et al. Two-year morbidity-mortality and alternatives to prolonged breast-feeding among children born to HIV-infected mothers in Côte d’Ivoire. PLoS Med. 2007;4:e17. doi: 10.1371/journal.pmed.0040017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taha TE, Kumwenda NI, Hoover DR, Kafulafula G, Fiscus SA, Nkhoma C, et al. The impact of breastfeeding on the health of HIV-positive mothers and their children in sub-Saharan Africa. Bull World Health Organ. 2006;84:546–554. doi: 10.2471/blt.05.027664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhn L, Aldrovandi GM, Sinkala M, Kankasa C, Semrau K, Mwiya M, et al. Effects of early, abrupt weaning on HIV-free survival of children in Zambia. N Engl J Med. 2008;359:130–141. doi: 10.1056/NEJMoa073788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kagaayi J, Gray RH, Brahmbhatt H, Kigozi G, Nalugoda F, Wabwire-Mangen F, et al. Survival of infants born to HIV-positive mothers, by feeding modality, in Rakai, Uganda. PLoS ONE. 2008;3:e3877. doi: 10.1371/journal.pone.0003877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carter RJ, Dugan K, El-Sadr WM, Myer L, Otieno J, Pungpapong N, et al. CD4+ cell count testing more effective than HIV disease clinical staging in identifying pregnant and postpartum women eligible for antiretroviral therapy in resource-limited settings. J Acquir Immune Defic Syndr. 2010;55:404–410. doi: 10.1097/QAI.0b013e3181e73f4b. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. [Accessed February 22, 2014];Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: towards universal access. 2010 at http://whqlibdoc.who.int/publications/2010/9789241599818_eng.pdf. [PubMed]
- 24.World Health Organization. Antiretroviral therapy of HIV infection in infants and children: towards universal access. [Accessed February 24, 2014];Recommendations for a public health approach. 2006 at http://www.who.int/hiv/pub/guidelines/art/en/index.html. [PubMed]
- 25.Iliff PJ, Piwoz EG, Tavengwa NV, Zunguza CD, Marinda ET, Nathoo KJ, et al. Early exclusive breastfeeding reduces the risk of postnatal HIV-1 transmission and increases HIV-free survival. AIDS. 2005;19:699–708. doi: 10.1097/01.aids.0000166093.16446.c9. [DOI] [PubMed] [Google Scholar]
- 26.Leroy V, Newell ML, Dabis F, Peckham C, Van de Perre P, Bulterys M, et al. International multicentre pooled analysis of late postnatal mother-to-child transmission of HIV-1 infection. Ghent International Working Group on Mother-to-Child Transmission of HIV. Lancet. 1998;352:597–600. doi: 10.1016/s0140-6736(98)01419-6. [DOI] [PubMed] [Google Scholar]
- 27.Coutsoudis A, Pillay K, Kuhn L, Spooner E, Tsai WY, Coovadia HM. Method of feeding and transmission of HIV-1 from mothers to children by 15 months of age: prospective cohort study from Durban, South Africa. AIDS. 2001;15:379–387. doi: 10.1097/00002030-200102160-00011. [DOI] [PubMed] [Google Scholar]
- 28.Vyankandondera J, Luchters S, Hassink E. Reducing risk of HIV-1 transmission from mother to infant through breastfeeding using antiretroviral prophylaxis in infants (SIMBA-study, Abstract N°LB7). 2nd International AIDS Society Conference on HIV pathogenesis, treatment and prevention; Paris, France. 2003. [Accessed February 22, 2013]. at http://www.iasociety.org/Default.aspx?pageId=11&abstractId=11061. [Google Scholar]
- 29.Palombi L, Marazzi MC, Voetberg A, Magid NA. Treatment acceleration program and the experience of the DREAM program in prevention of mother-to-child transmission of HIV. AIDS. 2007;21 (Suppl 4):S65–71. doi: 10.1097/01.aids.0000279708.09180.f5. [DOI] [PubMed] [Google Scholar]
- 30.Thomas TK, Masaba R, Borkowf CB, Ndivo R, Zeh C, Misore A, et al. Triple-antiretroviral prophylaxis to prevent mother-to-child HIV transmission through breastfeeding--the Kisumu Breastfeeding Study, Kenya: a clinical trial. PLoS Med. 2011;8:e1001015. doi: 10.1371/journal.pmed.1001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leroy V, Karon JM, Alioum A, Ekpini ER, Meda N, Greenberg AE, et al. Twenty-four month efficacy of a maternal short-course zidovudine regimen to prevent mother-to-child transmission of HIV-1 in West Africa. AIDS. 2002;16:631–641. doi: 10.1097/00002030-200203080-00016. [DOI] [PubMed] [Google Scholar]
- 32.Kesho Bora Study Group. Eighteen-month follow-up of HIV-1-infected mothers and their children enrolled in the Kesho Bora study observational cohorts. J Acquir Immune Defic Syndr. 2010;54:533–541. doi: 10.1097/QAI.0b013e3181e36634. [DOI] [PubMed] [Google Scholar]
- 33.Tonwe-Gold B, Ekouevi DK, Viho I, Amani-Bosse C, Toure S, Coffie PA, et al. Antiretroviral treatment and prevention of peripartum and postnatal HIV transmission in West Africa: evaluation of a two-tiered approach. PLoS Med. 2007;4:e257. doi: 10.1371/journal.pmed.0040257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rollins N, Mahy M, Becquet R, Kuhn L, Creek T, Mofenson L. Estimates of peripartum and postnatal mother-to-child transmission probabilities of HIV for use in Spectrum and other population-based models. Sex Transm Infect. 2012;88 (Suppl 2):i44–51. doi: 10.1136/sextrans-2012-050709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ciaranello AL, Perez F, Engelsmann B, Walensky RP, Mushavi A, Rusibamayila A, et al. Cost-effectiveness of World Health Organization 2010 guidelines for prevention of mother-to-child HIV transmission in Zimbabwe. Clin Infect Dis. 2013;56:430–446. doi: 10.1093/cid/cis858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–1243. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 37.Crampin AC, Floyd S, Glynn JR, Madise N, Nyondo A, Khondowe MM, et al. The long-term impact of HIV and orphanhood on the mortality and physical well-being of children in rural Malawi. AIDS. 2003;17:389–397. doi: 10.1097/00002030-200302140-00013. [DOI] [PubMed] [Google Scholar]
- 38.Nakiyingi JS, Bracher M, Whitworth JA, Ruberantwari A, Busingye J, Mbulaiteye SM, et al. Child survival in relation to mother’s HIV infection and survival: evidence from a Ugandan cohort study. AIDS. 2003;17:1827–1834. doi: 10.1097/00002030-200308150-00012. [DOI] [PubMed] [Google Scholar]
- 39.Zaba B, Whitworth J, Marston M, Nakiyingi J, Ruberantwari A, Urassa M, et al. HIV and mortality of mothers and children: evidence from cohort studies in Uganda, Tanzania, and Malawi. Epidemiology. 2005;16:275–280. doi: 10.1097/01.ede.0000155507.47884.43. [DOI] [PubMed] [Google Scholar]
- 40.Becquet R, Marston M, Dabis F, Moulton LH, Gray G, Coovadia HM, et al. Children who acquire HIV infection perinatally are at higher risk of early death than those acquiring infection through breastmilk: a meta-analysis. PLoS One. 2012;7:e28510. doi: 10.1371/journal.pone.0028510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Becquet R, Ekouevi DK, Menan H, Amani-Bosse C, Bequet L, Viho I, et al. Early mixed feeding and breastfeeding beyond 6 months increase the risk of postnatal HIV transmission: ANRS 1201/1202 Ditrame Plus, Abidjan, Côte d’Ivoire. Prev Med. 2008;47:27–33. doi: 10.1016/j.ypmed.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 42.Kuhn L, Sinkala M, Semrau K, Kankasa C, Kasonde P, Mwiya M, et al. Elevations in mortality associated with weaning persist into the second year of life among uninfected children born to HIV-infected mothers. Clin Infect Dis. 2010;50:437–444. doi: 10.1086/649886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.World Health Organization. Effect of breastfeeding on infant and child mortality due to infectious diseases in less developed countries: a pooled analysis. WHO Collaborative Study Team on the Role of Breastfeeding on the Prevention of Infant Mortality. Lancet. 2000;355:451–455. [PubMed] [Google Scholar]
- 44.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276:1253–1258. [PubMed] [Google Scholar]
- 45.Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD. Model parameter estimation and uncertainty: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force--6. Value Health. 2012;15:835–842. doi: 10.1016/j.jval.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 46.Leshabari SC, Blystad A, de Paoli M, Moland KM. HIV and infant feeding counselling: challenges faced by nurse-counsellors in northern Tanzania. Hum Resour Health. 2007;5:18. doi: 10.1186/1478-4491-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nachega JB, Uthman OA, Anderson J, Peltzer K, Wampold S, Cotton MF, et al. Adherence to antiretroviral therapy during and after pregnancy in low-, middle and high income countries: a systematic review and meta-analysis. AIDS. 2012;2012 doi: 10.1097/QAD.0b013e328359590f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohan D, Natureeba P, Plenty A, Luwedde F, Mwesigwa G, Ades V, et al. Efficacy and safety of LPV/r versus EFV in HIV+ pregnant and breast-feeding Ugandan women. 21st Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2014. URL not yet available. [Google Scholar]
- 49.Chipepo K, Nagot N, Meda N, Tumwine JK, Aku A, Jackson D, et al. Infant lopinavir/r versus 3TC to prevent postnatal HIV-1 transmission: The ANRS 12174 trial. 21st Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2014. URL not yet available. [Google Scholar]
- 50.Vaga BB, Moland KM, Evjen-Olsen B, Blystad A. Reflections on informed choice in resource-poor settings: The case of infant feeding counselling in PMTCT programmes in Tanzania. Soc Sci Med. 2014;105C:22–29. doi: 10.1016/j.socscimed.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 51.Chinkonde JR, Sundby J, de Paoli M, Thorsen VC. The difficulty with responding to policy changes for HIV and infant feeding in Malawi. Int Breastfeed J. 2010;5:11. doi: 10.1186/1746-4358-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zulliger R, Abrams EJ, Myer L. Diversity of influences on infant feeding strategies in women living with HIV in Cape Town, South Africa: a mixed methods study. Trop Med Int Health. 2013;18:1547–1554. doi: 10.1111/tmi.12212. [DOI] [PubMed] [Google Scholar]
- 53.Ross JS, Labbok MH. Modeling the effects of different infant feeding strategies on infant survival and mother-to-child transmission of HIV. Am J Public Health. 2004;94:1174–1180. doi: 10.2105/ajph.94.7.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Piwoz EG, Ross JS. Use of population-specific infant mortality rates to inform policy decisions regarding HIV and infant feeding. J Nutr. 2005;135:1113–1119. doi: 10.1093/jn/135.5.1113. [DOI] [PubMed] [Google Scholar]
- 55.Bertolli J, Hu DJ, Nieburg P, Macalalad A, Simonds RJ. Decision analysis to guide choice of interventions to reduce mother-to-child transmission of HIV. AIDS. 2003;17:2089–2098. doi: 10.1097/00002030-200309260-00010. [DOI] [PubMed] [Google Scholar]
- 56.Atashili J, Kalilani L, Seksaria V, Sickbert-Bennett EE. Potential impact of infant feeding recommendations on mortality and HIV-infection in children born to HIV-infected mothers in Africa: a simulation. BMC Infect Dis. 2008;8:66. doi: 10.1186/1471-2334-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuhn L, Kim HY, Walter J, Thea DM, Sinkala M, Mwiya M, et al. HIV-1 concentrations in human breast milk before and after weaning. Sci Transl Med. 2013;5:181ra151. doi: 10.1126/scitranslmed.3005113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuhn L, Aldrovandi G. Survival and health benefits of breastfeeding versus artificial feeding in infants of HIV-infected women: developing versus developed world. Clin Perinatol. 2010;37:843–862. doi: 10.1016/j.clp.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ciaranello AL, Park JE, Ramirez-Avila L, Freedberg KA, Walensky RP, Leroy V. Early infant HIV-1 diagnosis programs in resource-limited settings: opportunities for improved outcomes and more cost-effective interventions. BMC Med. 2011;9:59. doi: 10.1186/1741-7015-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.UNICEF, World Health Organization. Progress on Sanitation and Drinking Water 2013 Update. 2013. [Google Scholar]
- 61.UNICEF, World Health Organization. Progress on Drinking Water and Sanitation 2012 Update. 2012. [Google Scholar]
- 62.UNAIDS. [Accessed February 27, 2014];Report on the global AIDS epidemic 2013. 2013 at http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.