Figure 3.
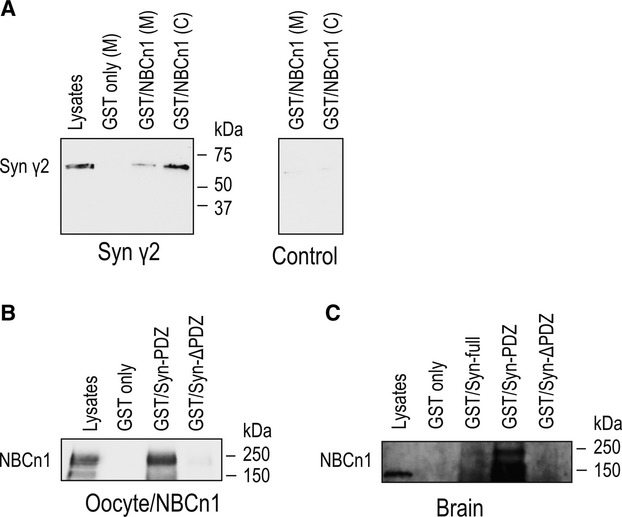
Interaction between NBCn1 and syntrophin γ2. (A) Lysates of Xenopus oocytes expressing syntrophin γ2 or none were incubated with GST/NBCn1 fusion proteins containing the C‐terminal amino acids of NBCn1. GST only served as a control. Pull‐down samples were immunoblotted with the syntrophin γ2 antibody. Syntrophin γ2 (60 kDa) was detected in pull–down samples from syntrophin γ2‐expressing oocytes, but not from control oocytes. Lysates were prepared from membrane (M) and cytosol (C). (B) Lysates of oocytes expressing rat NBCn1 were incubated with GST only, GST/Syn‐PDZ containing amino acid residues 1‐231 of syntrophin γ2, which include the PDZ domain, and GST/Syn‐ΔPDZ containing residues 232–539, which include the peckstrin homology domain. NBCn1 was pulled down by GST/Syn‐PDZ, but not by GST/Syn‐ΔPDZ. One of three experiments is shown. (C) Membrane lysates of rat brains were incubated with GST only, GST/Syn‐full containing the full‐length syntrophin γ2, GST/Syn‐PDZ, or GST/Syn‐ΔPDZ. NBCn1 was pulled down by GST/Syn‐full and GST/Syn‐PDZ, but not by GST/Syn‐ΔPDZ.
