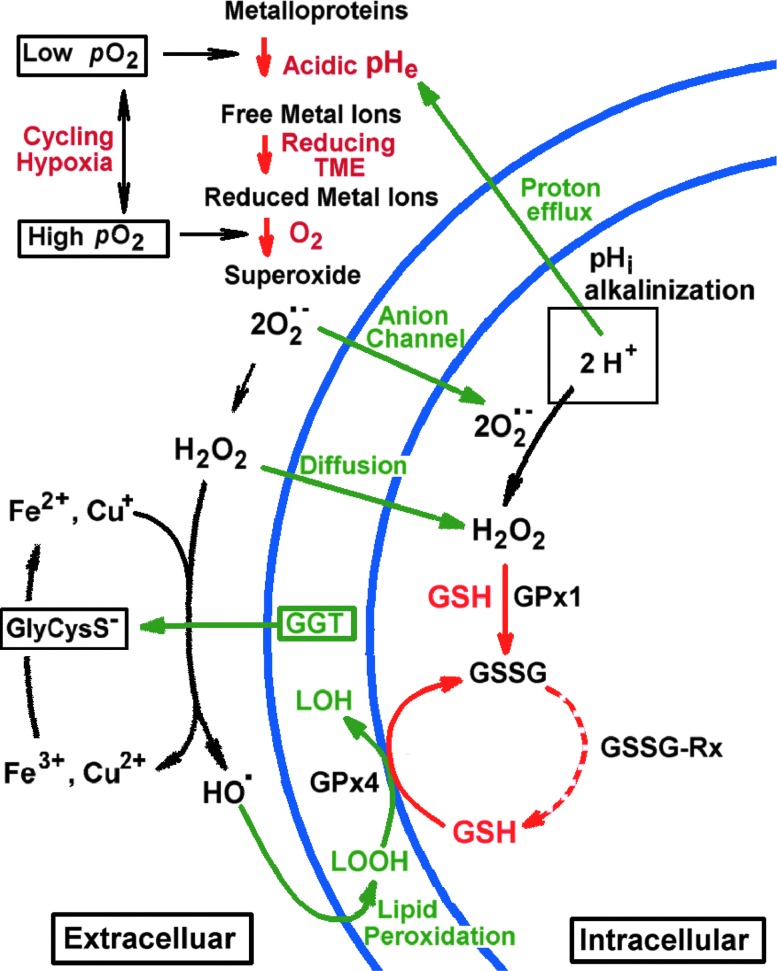
FIG. 3.
Janus-faced tumor redox microenvironment. The discussed key components of the TME, oxygen, pH, redox, and GSH, are shown in red. Hypoxia-induced acidosis potentiates accumulation of free metal ions such as Fe3+ and Cu2+. In its turn, a high reducing capacity of TME promotes metal ion reduction to the Fenton-active state, for example, via γ-glutamyltransferase (GGT)/GSH-dependent generation of reducing cysteinyl-glycine dipeptide, GlyCysS−. A cycling local hypoxia in TME facilitates further electron transfer to oxygen with formation of O2•− radical, triggering the radical reaction cascade of O2•− dismutation to H2O2 followed by OH-radical formation via the Fenton reaction. Low reactive oxygen species (ROS), H2O2, and O2•−, penetrate into tumor cells by diffusion or via anion channels, latter contributing to the increase of intracellular pH (pHi) (8, 37) with a corresponding decrease in oxidizing potential of ROS. In contrary, low acidic extracellular pH (pHe) in TME enhances ROS oxidizing potential toward surrounding cells that may result in oxidative damage and mutagenesis as well as adaptive response (e.g., increase of GSH) and changing cellular phenotypes. Increase in H2O2, O2•−, GSH, and pH has been shown contribute into the triggering cells in the proliferation stage (8). Compared with normal cells, cancer cells are well protected against oxidative stress, in part, by elevated GSH content and activities of GSH-dependent antioxidant enzymes, including glutathione peroxidases (GPx1 and GPx4 denote peroxidases that target hydrogen peroxide and lipid peroxide, correspondingly) and glutathione reductase (GSSG-Rx). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars