Abstract
Concurrent indoor and outdoor measurements of fine particulate matter (PM2.5) were conducted at three retirement homes in the Los Angeles Basin during two separate phases (cold and warm) between 2005 and 2006. Indoor-to-outdoor relationships of PM2.5 chemical constituents were determined and sources of indoor and outdoor PM2.5 were evaluated using a molecular marker-based chemical mass balance (MM-CMB) model. Indoor levels of elemental carbon (EC) along with metals and trace elements were found to be significantly affected by outdoor sources. EC, in particular, displayed very high indoor-to-outdoor (I/O) mass ratios accompanied by strong I/O correlations, illustrating the significant impact of outdoor sources on indoor levels of EC. Similarly, indoor levels of polycyclic aromatic hydrocarbons (PAHs), hopanes, and steranes were strongly correlated with their outdoor components and displayed I/O ratios close to unity. On the other hand, concentrations of n-alkanes and organic acids inside the retirement communities were dominated by indoor sources (e.g. food cooking and consumer products), as indicated by their I/O ratios, which exceeded unity. Source apportionment results revealed that vehicular emissions were the major contributor to both indoor and outdoor PM2.5, accounting for 39 and 46% of total mass, respectively. Moreover, the contribution of vehicular sources to indoor levels was generally comparable to its corresponding outdoor estimate. Other water-insoluble organic matter (other WIOM), which accounts for emissions from uncharacterized primary biogenic sources, displayed a wider range of contributions, varying from 2 to 73% of PM2.5, across all sites and phases of the study. Lastly, higher indoor than outdoor contribution of other water-soluble organic matter (other WSOM) was evident at some of the sites, suggesting the production of secondary aerosols as well as direct emissions from primary sources (including cleaning or other consumer products) at the indoor environments.
Keywords: PM2.5, Indoor air, Indoor-outdoor ratio, Source apportionment, Molecular marker-based chemical mass balance model, Los Angeles Basin
1. Introduction
Over the past decades, numerous epidemiological studies have reported consistent associations between exposure to particulate matter (PM) and a variety of adverse acute/chronic health effects including cardiovascular diseases (Brook et al., 2010), respiratory outcomes (Eisner et al., 2010; Holguin, 2008), and increased risk of adverse birth outcomes (Nieuwenhuijsen et al., 2013). The majority of outdoor air pollution studies largely relied on ambient air monitoring data from central sites located far from human subjects. Accordingly, air quality standards have been established for ambient environments, despite the fact that a large portion of human exposure to PM occurs indoors, where people spend most of their time (Jenkins et al., 1992; Klepeis et al., 2001). Considering the larger exposure time in different indoor micro-environments, the health effects of indoor air pollution of both indoor and outdoor origin is of considerable interest. Therefore, understanding the composition, behavior and sources of indoor PM and its relation to outdoor-generated PM are essential for personal exposure assessment.
In an occupied residential building, PM is emitted from several primary sources (such as cooking, sweeping, and other human activities), but could also be formed through the reactions of gas-phase precursors emitted both indoors and outdoors (i.e., secondary sources). Indoor PM concentrations are further affected by outdoor-generated PM penetrating indoors through convective flows (e.g. open doors and windows) or diffusional flows/infiltration (e.g. cracks and fissures) (Thatcher and Layton, 1995). Penetration of particles through the building cracks strongly depends on their size (Liu and Nazaroff, 2003; Rim et al., 2010). The results of a study conducted by Long et al. (2001) in 9 non-smoking homes of Boston, showed that ultrafine particles (UFP, particles with an aerodynamic diameter smaller than 0.1 μm) and coarse particles (PM2.5-10, particles with an aerodynamic diameter between 2.5 and 10 μm) have lower penetration efficiency compared to fine particles (PM2.5, particles with an aerodynamic diameter smaller than 2.5 μm). With the presence of indoor sources, indoor PM concentrations are often higher than their corresponding outdoor levels (Weschler and Shields, 1997). Their physical and chemical composition might also be significantly different (Lunden et al., 2003; Sarnat et al., 2006). Moreover, several epidemiological studies have demonstrated that exposure to indoor PM of outdoor origin is more deleterious compared with exposure to particles emitted indoors (Ebelt et al., 2005; Koenig et al., 2005) or exposure to the overall concentration of indoor PM (Delfino et al., 2008). Therefore, it is important to distinguish indoor from outdoor sources of PM in indoor environments, as this information is vital for both health risk assessment and proper regulatory guidelines for PM.
Only a few previous studies have attempted to estimate the contribution of specific sources to indoor PM using source apportionment techniques, including Positive Matrix Factorization (PMF) (Hopke et al., 2003; Larson et al., 2004; Minguillón et al., 2012; Ogulei et al., 2006), Chemical Mass Balance (CMB) model (Arhami et al., 2010; Kopperud et al., 2004), and Principal Component Analysis (PCA) (Koistinen et al., 2004).
The present study was carried out at three retirement homes in the Los Angeles Basin (LAB), as part of the Cardiovascular Health and Air Pollution Study (CHAPS), a cohort panel study investigating the health effects of micro-environmental exposure to PM on elderly retirees with a history of coronary artery disease (Delfino et al., 2009; Delfino et al, 2010). The objectives of the work presented in this paper are to: a) investigate the indoor/outdoor relationships of PM2.5 chemical constituents, b) identify major sources of fine PM in both indoor and outdoor environments, and c) quantify the contribution of each source to PM2.5 mass using an MM-CMB model.
2. Methodology
2.1. Sampling sites and periods
PM measurements were conducted at three retirement homes in the Los Angeles Basin (LAB), all located in the San Gabriel Valley, California. Site San Gabriel 1 (G1) was approximately 50 km east of Los Angeles, 3 km away from the nearest major freeway, located in a residential area. Site San Gabriel 2 (G2) was situated 8 km east of downtown Los Angeles, about 300 m south of a major freeway. Site San Gabriel 3 (G3) was located 55 km east of downtown Los Angeles, 2.5 and 0.15 km away from 2 busy freeways and a major street, respectively.
At each site, two identical sampling stations were set up, with each being located either indoors or outdoors. At G1, the indoor station was located in a recreational area of the community’s main building. The indoor sampling station at G2 was set up in the dining room of the community’s central building, while at G3 the indoor station was close to a gym and an activity room, located in the recreational area of the main community complex. All monitored homes prohibited smoking in these indoor environments. The outdoor sampling stations at all sites were set up in movable trailers, positioned about 300 m away from the indoor stations (Polidori et al., 2007).
Concurrent indoor and outdoor PM sampling was conducted at each site, during two separate phases between 2005 and 2006: warm phase (P1), including summer and early fall, and cold phase (P2), including late fall and winter. In each phase, 6 weeks of sampling were conducted at each location.
2.2. Instrumentation and chemical analysis
Size-segregated PM samples were collected daily (24-hour time-integrated) from Monday to Friday, using Sioutas Personal Cascade Impactor Samplers (Sioutas PCIS, SKC Inc., Eighty Four, PA, USA), operating at 9 lpm (Misra et al., 2002). Each PCIS was loaded with Zefluor filters (3 μm pore-size, Pall Life Sciences, Ann Arbor, Michigan, USA) and particles were collected in three size ranges, namely coarse (2.5 μm <dp<10 μm), accumulation (0.25 μm < dp < 2.5 μm), and quasi-ultrafine (dp<0.25 μm). The present study focuses only on fine PM (PM2.5), for which data from the accumulation (PM0.25-2.5) and quasi-ultrafine (PM0.25) PM modes were combined to derive PM2.5 concentrations. The PM mass concentrations were determined by pre- and post-weighting the Zefluor filters using a microbalance (Mettler Toledo Inc., Columbus, OH, USA), after equilibration under controlled laboratory conditions (temperature of 22–24 °C and relative humidity of 40–50%). A detailed description of the chemical analysis conducted on the Zefluor filters has been previously presented by Arhami et al. (2010). Briefly, filters were composited weekly (including 5 daily samples) and 92 different organic compounds were quantified by means of gas chromatography/mass spectrometry (GC/MS) (Stone et al., 2008). To measure the concentration of trace elements, sections of weekly-composited sample filters were microwave digested in an acid mixture (containing HNO3, HF and HCl) in Teflon vessels and digestates were then analyzed by high resolution magnetic sector Inductively Coupled Plasma Mass Spectrometry (SF-ICPMS, Thermo-Finnigan Element 2) (Herner et al., 2006). Water-soluble organic carbon (WSOC) was quantified using a Sievers Total Organic Carbon analyzer (General Electric Instruments; GE Analytical Instruments, Boulder, CO, USA) (Zhang et al., 2008).
Two semi-continuous OC-EC analyzers (Model 3F, Sunset Laboratory Inc.) were deployed at each site, one indoors and one outdoors, to measure the hourly mass concentration of elemental carbon (EC) and organic carbon (OC). A PM2.5 cyclone was placed at the inlet of the instruments to remove particles larger than 2.5 μm, and samples were collected at a nominal flow rate of 8 lpm. Also, a parallel plate diffusion denuder was placed upstream of each OC-EC instrument to remove most of the gas-phase OC, which is known to cause positive adsorption artifacts (Arhami et al., 2006).
2.3. Source apportionment
A molecular-marker based source apportionment model (MM-CMB) was used in this study to apportion PM2.5 organic carbon (OC) (Schauer et al., 1996). The model utilizes organic molecular markers that are source-specific tracers as fitting species and it was mathematically solved with an effective variance weighted least-squares solution (Watson et al., 1984), using the U.S. Environmental Protection Agency CMB (EPA-CMB8.2) software. Six sources were considered to have the highest contributions to fine OC in the sampling areas, including light-duty and heavy-duty vehicles (LDV and HDV, respectively) (Kam et al., 2012; Liacos et al., 2012), wood smoke (biomass burning in Western US) (Fine et al., 2004; Sheesley et al., 2007), ship emissions (Agrawal et al., 2008; Rogge et al., 1997), resuspended dust (Schauer, 1998), and vegetative detritus (Rogge et al., 1993). Vehicular emissions source profiles were based on recent on-road studies conducted at CA-110 and I-710 freeways in Los Angeles. However, inclusion of both LDV and HDV source profiles caused co-linearity in some CMB runs (29 cases). For these samples, the “estimable linear combinations of inestimable sources” was considered as the contribution from mobile sources (Watson et al., 1997), while for the rest of the samples, mobile source contributions were determined as the sum of both LDV and HDV source contributions (Lough et al., 2007). Moreover, to evaluate the sensitivity of the MM-CMB results to the selected vehicular emissions source profile, the contribution of mobile sources to fine OC was determined using a different source profile, derived from a roadway study conducted in 2005 at the CA-110 and I-710 freeways in Los Angeles (Kuhn et al., 2005; Ntziachristos et al., 2007b; Phuleria et al., 2007). The sensitivity analysis revealed the stability of the MM-CMB results for the estimated contributions from mobile sources (LDV+HDV) to PM2.5 OC, using the two considered source profiles, as indicated by the slope (±standard error) of 0.97 (±0.05) and R2 of 0.87 in Figure S1. A detailed discussion about this analysis and its results has been provided in the Supplementary Information.
A set of chemical species that are source-specific tracers and chemically stable during the transport from source to receptor was selected as the fitting species in the MM-CMB model. These species included EC, 22S-homohopane, 22R-homohopane, 17α(H)-21β(H)-hopane, 17α(H)-22,29,30-trisnorhopane, benzo(e)pyrene, benzo(b)fluoranthene, benzo(k)fluoranthene, benzo(ghi)perylene, levoglucosan, indeno(1,2,3-cd)pyrene, nonacosane, hentriacontane, tritriacontane, vanadium, nickel and aluminum.
The contributions from vegetative detritus were generally not statistically significantly different from zero, and were therefore removed from our calculations. Source contributions to total PM2.5 mass were evaluated by converting the MM-CMB results for fine OC to those of PM2.5 using the OC-to-PM mass ratios obtained from each source profile (Agrawal et al., 2008; Fine et al., 2004; Kam et al., 2012; Liacos et al., 2012; Rogge et al., 1993, 1997; Schauer, 1998; Sheesley et al., 2007). For samples displaying co-linearity for mobile sources, we assumed that the OC apportioned to mobile sources is entirely emitted from LDVs. The OC/PM ratio from the LDV source profile was therefore used for these co-linear samples. This assumption clearly has some uncertainties. To evaluate the range of variation in the mass apportionment of mobile sources for the co-linear cases, we conducted a sensitivity analysis under several different scenarios. The OC apportionment results were converted to PM mass-based assuming that the OC apportioned to mobile sources is emitted from 1) only HDV, 2) 25% LDV/75% HDV, 3) 75% LDV/25% HDV. Results were then compared to our prior assumption that OC from mobile sources is only emitted from LDVs. As can be seen in Table S1, results are about 25, 18, and 8% higher when assuming that OC apportioned to mobile sources is from only LDVs, compared to cases 1, 2 and 3, respectively.
In areas which are affected by the anthropogenic sources, ambient WSOC is mostly emitted from biomass burning sources (Docherty et al., 2008) or is formed through photochemical reactions (Weber et al., 2007). Other water-soluble organic carbon (other WSOC) is defined as the difference between measured total WSOC and WSOC from biomass burning (WSOCbb). WSOCbb was estimated as 71% of the OC apportioned to biomass burning from the CMB output (Sannigrahi et al., 2006). Other water-soluble organic matter (other WSOM) was then calculated by multiplying other WSOC by a factor of 1.8 (μgOM/μgOC) (Turpin and Lim, 2001). Other WSOM is mainly comprised of secondary organic aerosol (SOA), particularly in outdoor environments (Snyder et al., 2009), while it also includes the emissions from other primary sources in indoor environments such as organic acids from cleaning and other consumer products (Weschler, 2004).
Other water-insoluble organic matter (other WIOM) corresponds to water-insoluble OM that could not be apportioned to the considered primary sources. This was estimated by multiplying other water-insoluble organic carbon (other WIOC) also by a factor of 1.8 (Turpin and Lim, 2001). Other WIOC was determined as the difference between the total concentration of WIOC (OC-WSOC) and the sum of all primary source contribution estimates (excluding biomass burning), plus the concentration of WIOC from biomass burning. In central LA and Riverside, Heo et al. (2013) found that these compounds, in ambient PM2.5, mostly originate from primary biogenic sources such as food cooking or resuspended soil.
Lastly, since inorganic ions were not measured from the filters, sulfate was determined from the concentration of sulfur (S), assuming that all measured water soluble S by ICPMS is in the form of sulfate (Arhami et al., 2009). In addition to sources included in the OC apportionment, other WIOM, other WSOM and sulfate concentrations were considered in PM2.5 mass apportionment.
2.4. Meteorology and air exchange rates
Meteorological data, including temperature, relative humidity as well as wind speed and direction, sorted by study phases, sites, and indoor/outdoor locations are listed in Table S2. Mean indoor temperature showed very low variability across phases, while the average outdoor temperature was about 9 °C higher during the warm phase compared to the cold phase. Wind speed was generally higher during the warm phase, with a predominant westerly/southwesterly direction, which is typical of the LAB (Hasheminassab et al., 2013). Relative humidity showed moderate variation across sites, with slightly higher values during the warm phase compared to the cold phase (58.4±1.3% and 53.8±4.8%, respectively).
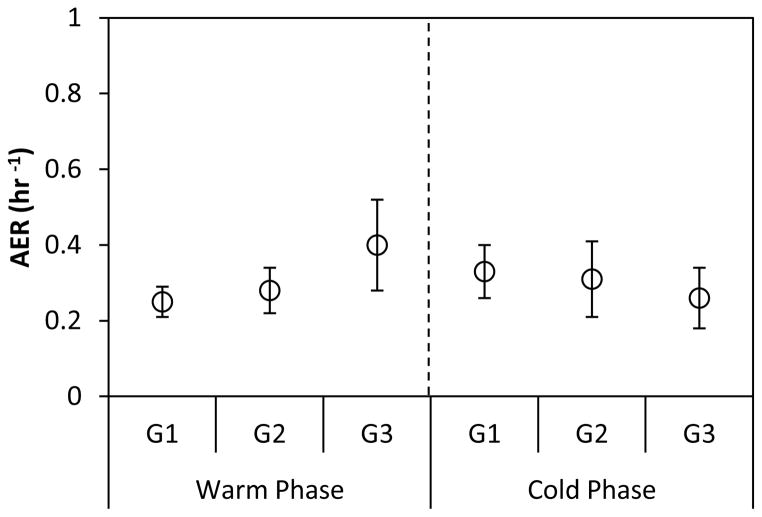
Polidori et al. (2007) estimated the air exchange rates ([AER] hr−1) in the studied homes by monitoring the decay of indoor CO during the periods affected by a dominant indoor source (such as food cooking). Figure 1 shows the average AERs at each site and phase of the study. The estimated AERs were generally low and relatively similar throughout the year at all sites. The low magnitude of AERs can be explained by the common use of air-conditioning along with the low number of open doors and windows in the studied retirement communities. In a study by Suh et al. (1994), conducted in 47 homes of State College, Pennsylvania, the median AERs measured in non-air-conditioned homes was about six times higher compared to air-conditioned homes. Also in the Boston area, during the summer, Long et al. (2000) reported that the measured AERs in non-air-conditioned homes were about 16 to 24 times of those AERs measured at a home equipped with a central air-conditioning system.
Figure 1.
Average indoor-outdoor air exchange rate ([AER] hr−1) at each site during the warm and cold phases. Error bars correspond to one standard deviation.
2.5. Data Analysis
As mentioned in preceding sections, chemical analyses were performed on weekly-composited filters, resulting in 6 sets of chemical data, and therefore estimated source contributions, at each site and phase of the study. To determine the indoor-to-outdoor (I/O) source relationships, I/O mass ratios and correlation coefficients (R) for given species were calculated for each study site and phase. The averages (±standard deviation) of these values are reported in the following sections. High overall correlation coefficient values indicate species originating from outdoors, whereas low or negative correlation coefficients and/or higher than unity I/O values indicate species produced by indoor sources (Daher et al., 2011).
In addition, for a specific phase, to investigate the statistical significance of the difference between indoor and outdoor levels of a given species (or a source), paired t-tests, at a 0.05 level of significance, were performed between each pair of datasets (N=6). When deviations from normality were observed in the data points, the significance of differences between the two datasets was evaluated by conducting the non-parametric Mann-Whitney rank sum test (U test), rather than the paired t-test (Brown and Hambley, 2002).
3. Results and discussions
3.1. Carbonaceous species
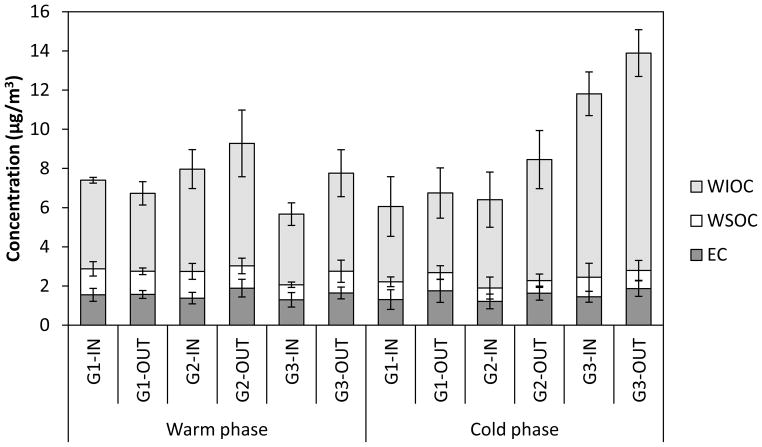
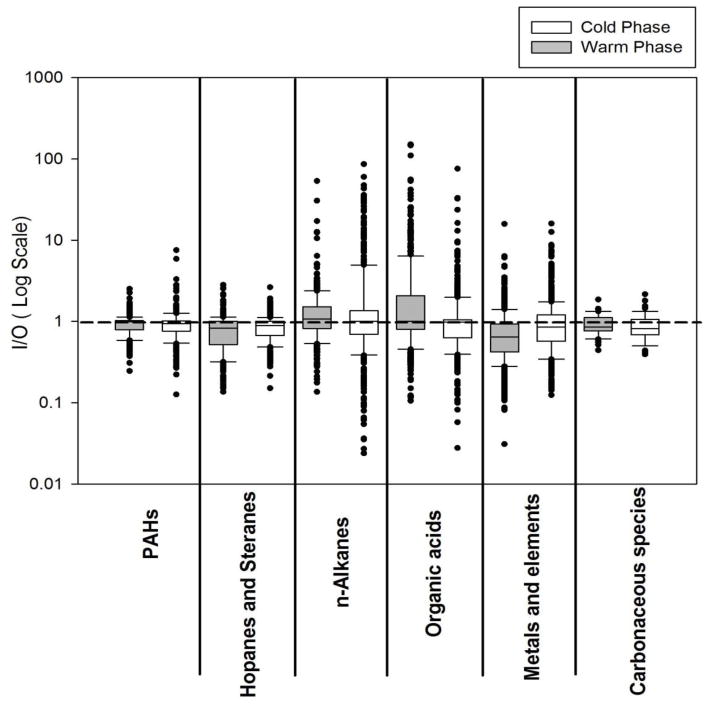
Average mass concentrations of carbonaceous species, including EC, WSOC, and WIOC, at all indoor and outdoor sampling sites, during both phases of the study, are presented in Figure 2 and summarized in Table S5. The variability of their weekly I/O ratios is illustrated within box plots in Figure 3. Their average I/O ratios as well as the I/O Pearson correlation coefficients (R) are presented in Table 1.
Figure 2.
Average indoor (IN) and outdoor (OUT) mass concentrations (μg/m3) of elemental carbon (EC), water-soluble organic carbon (WSOC), and water-insoluble organic carbon (WIOC) in the fine PM size fraction by site during the warm and cold phases. Error bars correspond to one standard deviation.
Figure 3.
Box plot of weekly indoor-to-outdoor (I/O) mass ratios for groups of individual organic compounds (including PAHs, Hopanes and steranes, n-alkanes, and organic acids), metals and elements, and carbonaceous species (EC, WSOC, WIOC) during the warm and cold phases. Each box represents the data for all 3 sites pooled together. The reference line shows the I/O mass ratio of 1.
Table 1.
Pearson correlation coefficients (R) and indoor-to-outdoor (I/O) mass ratios of elemental carbon (EC), water-soluble organic carbon (WSOC), water-insoluble organic carbon (WIOC), and selected metals and trace elements, averaged over all sites during the warm and cold phases. Errors correspond to one standard deviation.
| Species | Warm phase
|
Cold phase
|
||||||
|---|---|---|---|---|---|---|---|---|
| I/O | ± | R | ± | I/O | ± | R | ± | |
| EC | 0.82 | 0.14 | 0.80 | 0.04 | 0.77 | 0.15 | 0.86 | 0.16 |
| WSOC | 1.07 | 0.37 | 0.70 | 0.18 | 1.08 | 0.51 | 0.43 | 0.70 |
| WIOC | 0.89 | 0.20 | 0.47 | 0.59 | 0.84 | 0.23 | 0.34 | 0.55 |
|
| ||||||||
| Mg | 0.58 | 0.25 | 0.52 | 0.49 | 0.81 | 0.34 | 0.34 | 0.56 |
| Al | 0.62 | 0.31 | 0.22 | 0.30 | 1.18 | 0.74 | 0.10 | 0.72 |
| S | 0.74 | 0.22 | 0.75 | 0.29 | 1.15 | 1.07 | 0.49 | 0.75 |
| K | 0.97 | 0.59 | 0.30 | 0.46 | 1.08 | 0.45 | 0.09 | 0.16 |
| Ca | 0.79 | 0.33 | 0.44 | 0.41 | 1.13 | 0.76 | 0.31 | 0.68 |
| Ti | 0.67 | 0.33 | 0.36 | 0.53 | 0.78 | 0.29 | 0.30 | 0.62 |
| V | 0.77 | 0.19 | 0.77 | 0.22 | 0.90 | 0.50 | 0.42 | 0.64 |
| Cr | 1.00 | 0.74 | 0.72 | 0.25 | 0.97 | 0.48 | −0.10 | 0.14 |
| Mn | 0.78 | 0.41 | 0.28 | 0.22 | 0.92 | 0.51 | 0.45 | 0.76 |
| Fe | 0.80 | 0.42 | 0.33 | 0.57 | 0.92 | 0.40 | 0.60 | 0.49 |
| Ni | 0.94 | 0.61 | 0.75 | 0.22 | 0.86 | 0.28 | 0.39 | 0.73 |
| Cu | 0.68 | 0.46 | −0.08 | 0.33 | 1.00 | 0.65 | 0.14 | 0.75 |
| Zn | 0.75 | 0.21 | 0.47 | 0.50 | 1.12 | 0.38 | 0.71 | 0.10 |
| Pb | 0.61 | 0.23 | 0.62 | 0.28 | 0.88 | 0.36 | 0.46 | 0.39 |
I/O ratios for carbonaceous species showed very low variability during both phases of the study (Figure 3), indicating the relatively common origins of these species across all 3 sites. EC, which mainly originates from incomplete combustion of fossil fuels and is a tracer of pollution from diesel exhaust (Schauer, 2003), constituted a small fraction of PM2.5, with an average contribution of 8.7±2.8% to total mass, over all sites and both phases. In the RIOPA (Relationship of Indoor, Outdoor, and Personal Air) study, which was conducted in 105 homes of Los Angeles between 1999 and 2001, Polidori et al. (2006) reported that EC constituted about 7% of PM2.5 mass concentration in both indoor and outdoor environments, which is generally in agreement with the ratios reported in this study. EC did not show any significant seasonality, but its indoor concentration was comparable if somewhat lower than that outdoors, with average I/O ratios of 0.82 and 0.77 during the warm and cold phases, respectively. These I/O ratios were accompanied by high R-values (about 0.83 on average during both phases), suggesting that a substantial fraction of indoor EC is attributed to that of outdoor concentrations which infiltrated indoors. Our results are very similar to those reported by Geller et al. (2002). They reported an average I/O ratio of 0.85 and R-value of 0.84 for EC in 13 residences in Coachella Valley, California, during the winter and spring.
OC accounted for about 44 and 33% of PM2.5 mass indoors and outdoors, respectively, with levels ranging from 3.1 to 13.0 μg/m3, across all sites and both study phases. These contributions were relatively higher than those reported in the RIOPA study. Polidori et al. (2006) found that contributions of OC to PM2.5 in indoor and outdoor environments of 105 homes of Los Angeles were around 34.4 and 21.0%, respectively. It can be readily inferred from Figure 2 that OC was predominantly water-insoluble (83.1±7.5%). As can be seen in Table 1, WIOC displayed high I/O ratios with relatively low R-values (0.34–0.47) during both phases of the study, suggesting the presence of important indoor primary sources, such as food cooking, cleaning products, and organic dusts. Outdoor WSOC, as an indicator of SOA formation (Weber et al., 2007), displayed slightly higher concentrations during the warm phase (1.14±0.04 μg/m3) compared to the cold phase (0.83±0.17 μg/m3), mainly because of higher photochemical activities coupled with increased advection of aged particles from upwind “source” regions, during the warmer months (Sardar et al., 2005). While WSOC exhibited I/O ratios higher than 1 during both phases, its R-value was significantly higher during the warm phase, compared to the cold phase (0.7 and 0.43, respectively). These results reflect a significant impact of outdoor sources on indoor levels of WSOC during the warmer months, while they support the predominance of indoor sources (both primary and secondary to a lesser extent) in indoor environments during the cold seasons.
3.2. Metals and trace elements
The variability of weekly I/O ratios for all 47 detected metals and trace elements (TEs) across all sites is shown within box plots for each phase of the study in Figure 3. The average I/O ratios and the correlation coefficients (R) of selected metals and TEs at each site and phase of the study are also presented in Table 1, and concentrations tabulated in Table S5. These selected species were among the most abundant elements and are toxicologically important (Oeder et al., 2012; Shi et al., 2003; Valavanidis et al., 2005). It should be noted that at some sites and phases of the study, concentrations of few metals and TEs were not detectable indoors or outdoors. To avoid obtaining inestimable indoor-to-outdoor (I/O) mass ratios, the concentration of these species were assumed as half of their limit of detection (LOD). LODs for non-detectable species ranged from 3.45 × 10−4 to 80.27 and 5.64 × 10−4 to 11.19 ng/m3 in the ultrafine and accumulation modes, respectively.
The box plots indicate a wide range of variability in the I/O ratios of metals and TEs during both study phases (Figure 3), suggesting that these species are emitted from a broad range of sources. Of all the inorganic elements, S was the most abundant species at all sites and phases of the study, with levels ranging from 392 to 1930 ng/m3. The outdoor concentration of S was on average 2.7 times higher during the warm phase, compared to the cold phase. Higher concentration of S during the warm phase indicates that S was mostly in the form of sulfate during the warmer months (Arhami et al., 2009). Average I/O ratios were generally below 1, except for few species during the cold phase (namely, Al, S, Ca, K, and Zn), which exceeded unity. During the warm phase, indoor concentrations of S, V, Cr, and Ni showed relatively high correlations with their corresponding outdoor levels (R-values ranging from 0.72 to 0.77), indicating a significant influence of outdoor sources on the indoor levels of these species. Vanadium and sulfur are mostly associated with ship emissions, refinery operations, and residual oil combustion (Arhami et al., 2009), while Ni and Cr are known as tracers of industrial emissions (Ntziachristos et al., 2007a; Singh et al., 2002). The average I/O ratio for Zn was 0.75±0.21 and 1.12±38 during the warm and cold phases, respectively. While Zn primarily originates from outdoor sources, smoking has been found to be a dominant source of Zn at indoor environments (Jones et al., 2000). Given that the studied homes were non-smoking residences, increased indoor concentration of Zn is mostly attributed to the enhanced infiltration of these particles from outdoors. Another anthropogenically-dominated metal, Cu, showed high average I/O ratios during the warm and cold phases (0.68 and 1.00, respectively), while it was weakly correlated with its corresponding outdoor concentrations (R-values ranged from −0.08 to 0.14), indicating the presence of potential indoor sources such as resuspended mineral dust (Ibanez et al., 2012) or emissions from indoor electric universal motors such as those inside vacuum cleaners, toys, hair dryers, and blenders (Szymczak et al., 2007). More detailed discussion on indoor/outdoor relationship of size-fractionated metals and TEs at all sampling sites has been provided by Polidori et al. (2009).
3.3. Organic compounds
Individual organic constituents of PM2.5 were grouped into polycyclic aromatic hydrocarbons (PAHs), hopanes and steranes, n-alkanes, and organic acids. As implemented with the inorganic elements, concentrations of non-detectable organics were assumed as half of their LODs. LODs for non-detectable species ranged from 1.46 × 10−2 to 0.96 and 6.81 × 10−3 to 2.92 ng/m3 in the ultrafine and accumulation modes, respectively. Table S5 presents the concentrations of selected organic species, which were used as fitting species in the MM-CMB model.
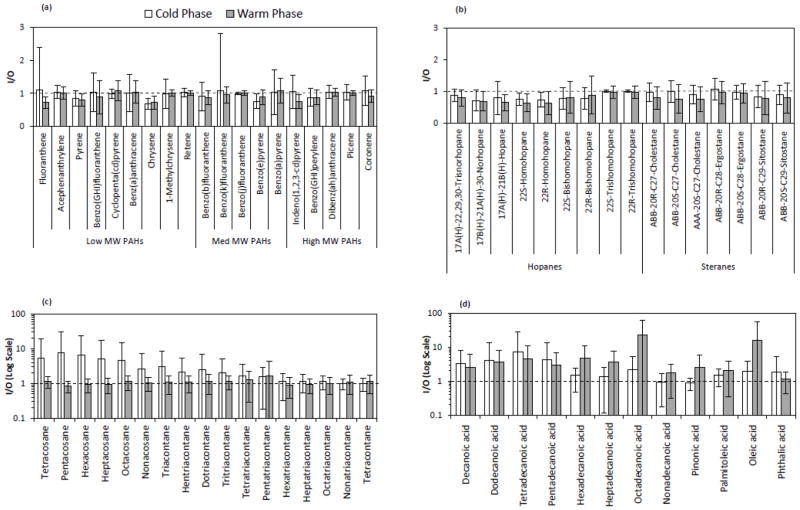
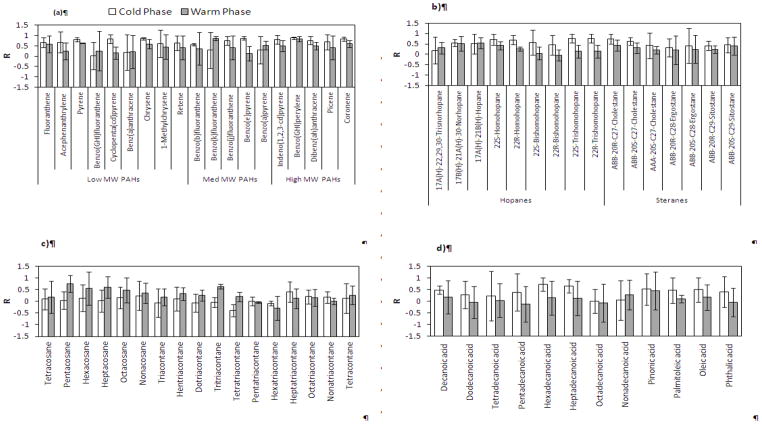
Figure 3 shows the box plot distributions of weekly I/O mass ratios for groups of individual organic species (including 19 PAHs, 16 hopanes and steranes, 27 n-alkanes, and 41 organic acids), during the warm and cold phases. Less variability was observed for PAHs, hopanes and steranes, suggesting that these species originate from rather similar sources across all sites, most likely vehicular emissions (Lough et al., 2007). The median I/O values for PAHs ranged from 0.94 to 0.99 during the cold and warm phases, respectively. The I/O ratios showed slightly lower values for hopanes and steranes with median levels ranging from 0.82 during the warm phase to 0.89 during the cold phase. The measured I/O values for individual PAHs and hopanes are well within the ranges reported in previous studies for the fine PM size fraction. Olson et al. (2008) reported median I/O ratios ranging from 0.7 to 1 for 7 PAHs and 1 to 1.1 for 3 hopanes in Tampa, Florida. Ohura et al. (2004) also reported median I/O ratios ranging from 0.62 to 1.27 during the summer and 0.27 to 1.09 during the winter for 19 individual PAHs in two different cities of Japan. Unlike PAHs, hopanes and steranes, n-alkanes and organic acids showed a broader range of I/O ratios, accompanied by median values slightly higher than 1, indicating a larger variability in their sources of origin in the indoor and outdoor environments over different sites.
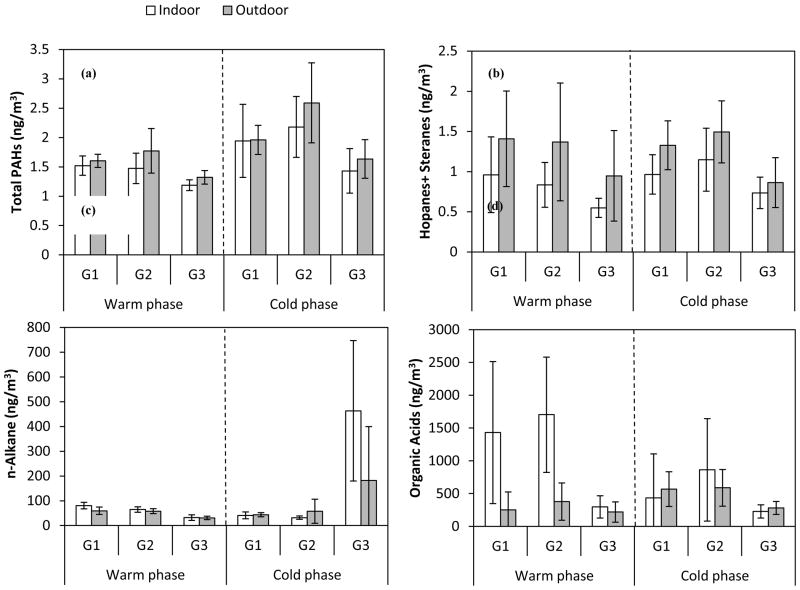
Figure 4a–d shows the average concentration of organic compounds in both indoor and outdoor environments, at each site and phase of the study. Moreover, the average I/O mass ratios and I/O Pearson correlation coefficients (R) were determined for selected organic species in both phases across all sites, as shown respectively in Figures 5a–d and 6 a–d.
Figure 4.
a–d. Average mass concentrations (ng/m3) of total (a) PAHs, (b) hopanes and steranes, (c) n-alkanes, and (d) organic acids in indoor and outdoor environments at each site during the warm and cold phases. Error bars correspond to one standard deviation.
Figure 5.
a–d. Indoor-to-outdoor (I/O) mass ratios of selected (a) PAHs, (b) hopanes and steranes, (c) n-alkanes, and (d) organic acids, averaged over all sites during the warm and cold phases. Error bars correspond to one standard deviation. The reference line shows the I/O mass ratio of 1.
PAHs are typically produced from incomplete combustion of fossil fuels and/or other organic matter, such as cooking at indoor environments (Abdullahi et al., 2013; Manchester-Neesvig et al., 2003). Average mass concentrations of total PAHs were overall higher during the cold phase (Figure 4a), mainly due to higher atmospheric stability and lower degree of dispersion and mixing during the colder seasons. On the other hand, enhanced photo-degradation of PAHs (Miet et al., 2009) along with increased partitioning to the gas phase at higher temperatures (Mader and Pankow, 2002), can result in lower outdoor concentrations of these species during the warm phase. The highest average outdoor concentration of total PAHs in both phases was observed at G2, which was the closest sampling location to a major freeway among all sites. The average indoor concentrations of total PAHs were not statistically significantly different than the outdoor levels at all sites and both phases of the study (p values ranged from 0.06 to 0.95), suggesting likely contribution of outdoor sources to indoor particle levels. This was further corroborated by the I/O mass ratios (Figure 5a) and correlation coefficients for individual PAHs (Figure 6a). PAH components displayed average I/O ratios close to unity, with generally high and positive correlation coefficients (median R-value across components is 0.61 and 0.77 during the warm and cold phase, respectively), indicating a strong impact from outdoor sources (most notably diesel and gasoline exhaust) on indoor levels of PAHs. Potential indoor sources of PAHs include smoking, gas cooking and heating appliances (Liu et al., 2001; Ohura et al., 2002; Orasche et al., 2012). However, since the retirement communities in this study were non-smoking residences, the contribution of PAHs from tobacco smoke is unlikely (Arhami et al., 2010).
Figure 6.
a–d. Pearson correlation coefficients of selected (a) PAHs, (b) hopanes and steranes, (c) n-alkanes, and (d) organic acids, averaged over all sites during the warm and cold phases. Error bars correspond to one standard deviation.
Unlike PAHs, the summed concentration of hopanes and steranes exhibited less seasonality at all sites (Figure 4b). This is likely due to the lower volatility and reactivity of these compounds compared to PAHs (Ruehl et al., 2011). Moreover, given that hopanes and steranes primarily originate from lubricating oil of gasoline- and diesel-powered vehicles, their emission rates are relatively insensitive to driving conditions (Lough et al., 2007). As can be inferred from Figure 5b, the average I/O ratios of individual hopanes were slightly lower than those for steranes, yet both were close to unity (ranging from a minimum of 0.6 to a maximum of 1.1, for all components across both phases). Seasonally, the I/O ratios were relatively higher during the cold phase accompanied by larger R-values compared to the warm phase (median R-values for all components during the warm and cold phases are 0.27 and 0.64, respectively). These results indicate the strong influence of outdoor sources on indoor levels of hopanes and steranes, particularly during the cold seasons.
The average cumulative concentration of measured n-alkanes was higher indoors than outdoors, with much higher levels at G3 during the cold phase. While the average I/O ratios for individual n-alkanes (C24-C40) during the warm phase spanned around unity (min=0.9, max= 1.7, median= 1.1), these values significantly increased (up to 8) during the cold phase. Furthermore, I/O correlation coefficients showed overall greater and/or positive values during the warm phase compared to the cold phase. These results, altogether, are indicative of a considerable influence of indoor sources (e.g. cooking, household products, dust, and candle burning (Fine et al., 1999; Kleeman, 2008; Schauer, 1999)) on the indoor levels of these species, particularly during the cold phase. The carbon preference index (CPI) of n-alkanes (C19-C40) was calculated for all sites and phases (Figure S2) to further investigate the origins of these species. CPI is defined as the ratio of summed odd-carbon number n-alkanes to even-carbon number n-alkanes (Simoneit, 1986). CPI around 1 indicates the predominance of emissions from anthropogenic sources, while emissions from biogenic sources usually exhibit CPI greater than 2 (Daher et al., 2011). Both indoor and outdoor n-alkanes showed CPI values around unity (CPI ranging from 0.73 to 0.94), suggesting an overall prevalence of anthropogenic sources in both indoor and outdoor environments.
The average total concentration of measured organic acids was overall substantially higher indoors than outdoors (Figure 4d). At G1 and G2 during the warm phase, in particular, average indoor concentrations of total organic acids were significantly higher (more than 4 times) than their corresponding outdoor levels, with p values of 0.015 and 0.006, respectively. The I/O correlation coefficients for individual organic acids were typically low (or negative) along with high standard deviation, while they displayed I/O ratios significantly higher than 1 in both phases (Figure 5d). These results confirm that the indoor levels of these species are strongly affected by indoor sources, potentially including emissions from human skin (Nicolaides, 1974), wax emission from painted walls (Naik et al., 1991), and food cooking (Abdullahi et al., 2013).
3.4. Source apportionment of PM2.5
As noted in the methodology section, OC apportionment results from the MM-CMB model were converted to PM mass-based results using OC/PM ratios obtained from each source profile (Agrawal et al., 2008; Fine et al., 2004; Kam et al., 2012; Liacos et al., 2012; Rogge et al., 1993, 1997; Schauer, 1998; Sheesley et al., 2007). In addition to the source contributions estimated by MM-CMB, other WSOM, other WIOM, and sulfate were considered as contributors to PM2.5 mass concentration. Source apportionment results for PM2.5 OC and PM2.5 mass concentrations are respectively illustrated in Figure S3 and Figure 7, and also summarized in Tables S3 and S4. We should note that some samples were affected by positive OC adsorption artifacts during two weeks of sampling in the cold study phase at G3. The source apportionment results for these two weeks were excluded from our calculations.
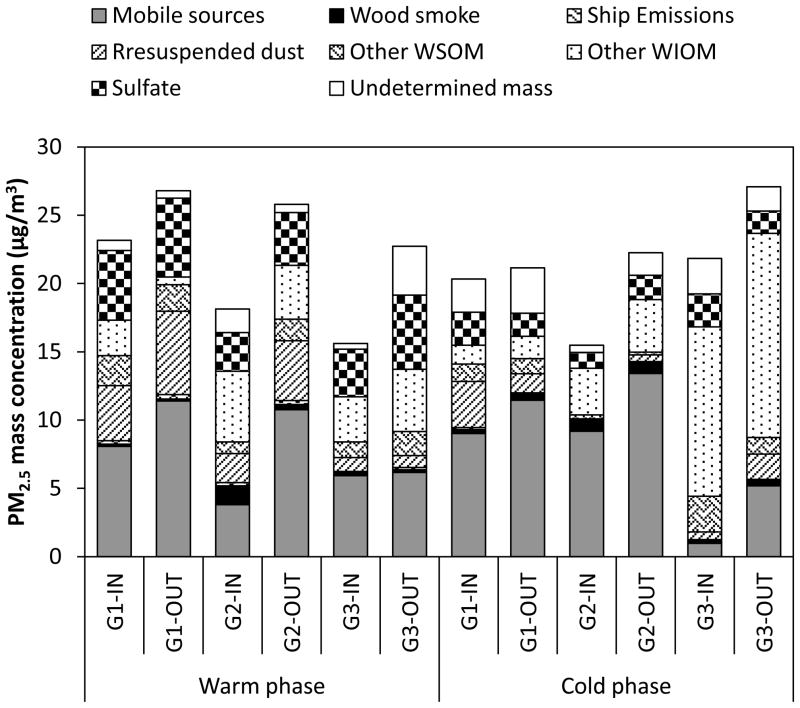
Figure 7.
Average relative contribution of different sources to PM2.5 mass concentration (μg/m3) in indoor (IN) and outdoor (OUT) environments at each site during the warm and cold phases.
Overall, mobile (vehicular) sources were found to be the major contributors to fine PM, accounting for 39±21 and 46±17% of PM2.5 mass, respectively at indoor and outdoor environments, averaged across all sites and both phases. Their contributions to PM2.5 displayed an average I/O ratio of 0.74±0.34 over all sites and phases, illustrating a significant influence of outdoor mobile source emissions on indoor levels of PM2.5. This was further supported by the results of the statistical analyses, which showed that indoor and outdoor estimated contributions from mobile sources were not statistically significantly different (p values ranged from 0.13 to 0.91) at all sites and both phases of the study (with exception of G2 during the warm phase, p= 0.014). In the RIOPA study, Meng et al. (2007) applied positive matrix factorization (PMF) receptor model on paired indoor and outdoor PM2.5 species concentrations to characterize and quantify sources of indoor and outdoor PM2.5. They reported an I/O ratio of about 0.6 for vehicular emission source contributions for 105 homes in Los Angeles, which is within the range of our findings (i.e. 0.4 to 1). In another source apportionment study conducted by Minguillón et al. (2012) in 54 homes of Barcelona, the median I/O ratio for the estimated source contributions from mobile sources was about 0.75, which is quite similar to our results (i.e. 0.74±0.34).
Except for G3 during the cold phase, other WIOM, which represents uncharacterized primary sources, on average accounted for 20 and 14% of PM2.5 in indoor and outdoor environments, respectively, over all sites and both study phases. For ambient PM2.5, in central LA and Riverside, Heo et al. (2013) reported that these compounds mainly originate from primary biogenic sources such as food cooking or resuspended soil, which were not considered in the CMB model. Other WIOM displayed a significant contribution to PM2.5 at G3 during the cold phase, with levels ranging from 10.0 to 16.1 μg/m3 and 10.1 to 16.9 μg/m3 at indoor and outdoor environments, respectively. The high contribution of other WIOM at G3 during the cold phase suggests the presence of a very unique source at this site that also impacted the levels of n-alkanes, as shown earlier.
The average contribution of sulfate to PM2.5 was 18% at indoor sites and 15% at outdoor environments. Its outdoor contribution was generally greater during the warm phase (5.0±1.0 μg/m3 or 23±6% of PM2.5) compared to the cold phase (1.7±0.1 μg/m3 or 7.9±1.4% of PM2.5), which is consistent with the increased sulfate formation as a result of enhanced photochemical activity during warmer seasons (Khoder, 2002).
Averaged over all sites and both phases, source contribution estimate of resuspended dust was 2.2±1.9 μg/m3, corresponding to 11±8% of fine PM. Seasonally, the average contribution of resuspended dust at indoor and outdoor environments was respectively 1.8 and 3.0 times higher in the warm phase compared to the cold phase. Occupant-related activities and road dust are the major sources of resuspended dust in indoor and outdoor environments, respectively.
Other WSOM showed significant seasonality in outdoor environments, with higher contribution to PM2.5 during the warm phase (1.8±0.2 μg/m3 or 7.6±0.7% of PM2.5) compared to the cold phase (0.8±0.6 μg/m3 3.8±2.6% of PM2.5). This trend in outdoor environments is most likely due to increased photochemical production of WSOM in the atmosphere during warmer months (Sardar et al., 2005). The indoor contribution of other WSOM ranged from 0.3 to 2.6 μg/m3 (or 1 to 13% of PM2.5) over all sites and phases. Indoor concentrations of other WSOM were higher than outdoors, at all sites during the cold phase and at G1 during the warm phase. Nonetheless, none of these elevations were statistically significant (p values ranged from 0.15 to 0.70). Several studies have shown that ozone-initiated reactions with emissions from consumer products (Sarwar et al., 2004), building materials (Aoki and Tanabe, 2007), and cleaning products (Singer et al., 2006), lead to SOA formation in indoor environments. Moreover, as noted earlier, indoor other WSOM is also impacted by primary sources such as organic acids from cleaning and other consumer products (Weschler, 2004).
Wood smoke accounted for about 3% of PM2.5 mass, averaged over all sites and phases. Outdoor concentrations of wood smoke displayed a significant seasonality, with more than 2 times higher contribution to ambient PM2.5 during the cold phase compared with the warm period (with the exception of G2). This trend is most likely due to increased wood burning for domestic heating purposes during the cold season. Ship emissions were the most minor primary source of PM2.5, with less than 1% contribution to total mass, averaged over all sites and both phases of the study.
Lastly, un-apportioned PM mass accounted for just 7±5% of measured PM2.5, on average. In most of the cases, the un-apportioned PM mass concentrations were not statistically different from zero. This small discrepancy in mass apportionment could be in part associated with ammonium nitrate, which was not measured in this study, but could constitute an important component of PM2.5 (Hughes et al., 2002), especially in outdoor environments. Additionally, uncertainties in the source profiles composition and multiplication factors used to estimate other WIOM and other WSOM could lead to this discrepancy.
4. Conclusions
To investigate the indoor/outdoor relationships of PM2.5 and its chemical constituents, as well as to identify and quantify major sources of PM2.5 in indoor and outdoor environments, a sampling campaign was conducted between 2005 and 2006 at three retirement homes in the Los Angeles Basin. Outdoor PM2.5 levels were constantly higher than those measured indoors at all sites and phases of the study. Indoor concentrations of EC were comparable to their corresponding outdoor levels (average I/O= 0.80) and were strongly correlated (average R= 0.83), indicating a considerable impact of outdoor sources on the indoor levels of EC. Indoor levels of metals and trace elements were found to be mostly affected by outdoor sources. PAHs, hopanes and steranes, exhibited low variability in their indoor-to-outdoor (I/O) mass ratios, with median values close to unity, accompanied by relatively high I/O correlations, reflecting the significant impact of outdoor sources (most notably vehicular emissions) on their indoor levels. By contrast, n-alkanes and organic acids exhibited much higher I/O ratios along with poor correlations with their corresponding outdoor levels, implying that the indoor concentrations of these species were mostly dominated by indoor sources.
Source apportionment results revealed that vehicular sources were the dominant sources of PM2.5, with an average contribution of 43±19% over all sites and phases of the study. Moreover, the contribution of mobile sources to indoor PM levels was generally comparable to their corresponding outdoor estimates (I/O= 0.74±0.34, averaged over all sites and phases). Except for G3 during the cold phase, sulfate was generally the next most abundant component, across all sites and phases. Other WIOM, which accounts for uncharacterized primary biogenic sources, showed significant contributions to indoor and outdoor PM2.5 during the cold phase at G3 (71.5 and 73.4%, respectively). Resuspended dust and other WSOM respectively contributed to 11 and 7% of PM2.5, on average across all sites and phases.
Our results suggest that even though the elderly subjects of this study spend most of their time in indoor micro-environments with relatively low air exchange rates, they are exposed to considerable levels of PM of both indoor and outdoor origin. Therefore, a full understanding of the adverse health effects of exposure to indoor PM requires a detailed knowledge of human activity patterns in different micro-environments and, more importantly, information on the degree to which indoor and outdoor sources contribute to indoor levels of PM.
Supplementary Material
Highlights.
Concurrent indoor and outdoor PM2.5 measurement was conducted in three homes.
Indoor n-alkanes and n-alkanoic acids were strongly influenced by indoor sources.
Indoor metals, PAHs, hopanes and steranes were strongly impacted by outdoor sources.
Molecular marker-based chemical mass balance model was used.
Vehicular emissions were found to be the major contributor to indoor and outdoor PM2.5.
Acknowledgments
This research was funded in part by NIEHS-NIH (Grant no. ES-012243) and the California Air Resources Board (ARB) (contracts no. 03-329 and 09-341). The study on the accumulation mode of the samples was funded by ARB (contract no. 09-341) and is presented in the final report. We would like to acknowledge the staff at the Wisconsin State Laboratory of Hygiene for their assistance with the chemical analysis. We also wish to acknowledge the support of USC Provost’s Ph.D. fellowship.
Footnotes
Conflict of interest
C.S receives royalties from SKC Inc. for the Sioutas Personal Cascade Impactor air sampling device used in this study. The remaining authors declare no conflicts of interest, financial or otherwise.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdullahi KL, Delgado-Saborit JM, Harrison RM. Emissions and indoor concentrations of particulate matter and its specific chemical components from cooking: A review. Atmospheric Environment. 2013;71:260–294. [Google Scholar]
- Agrawal H, Malloy QGJ, Welch WA, Wayne Miller J, Cocker DR., III In-use gaseous and particulate matter emissions from a modern ocean going container vessel. Atmospheric Environment. 2008;42:5504–5510. [Google Scholar]
- Aoki T, Tanabe S-i. Generation of sub-micron particles and secondary pollutants from building materials by ozone reaction. Atmospheric Environment. 2007;41:3139–3150. [Google Scholar]
- Arhami M, Kuhn T, Fine PM, Delfino RJ, Sioutas C. Effects of Sampling Artifacts and Operating Parameters on the Performance of a Semicontinuous Particulate Elemental Carbon/Organic Carbon Monitor. Environmental Science & Technology. 2006;40:945–954. doi: 10.1021/es0510313. [DOI] [PubMed] [Google Scholar]
- Arhami M, Minguillón MC, Polidori A, Schauer JJ, Delfino RJ, Sioutas C. Organic compound characterization and source apportionment of indoor and outdoor quasi-ultrafine particulate matter in retirement homes of the Los Angeles Basin. Indoor Air. 2010;20:17–30. doi: 10.1111/j.1600-0668.2009.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arhami M, Sillanpää M, Hu S, Olson MR, Schauer JJ, Sioutas C. Size-Segregated Inorganic and Organic Components of PM in the Communities of the Los Angeles Harbor. Aerosol Science and Technology. 2009;43:145–160. [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC, Whitsel L, Kaufman JD. Particulate Matter Air Pollution and Cardiovascular Disease An Update to the Scientific Statement From the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Brown PMBLC, Hambley DF. Statistics for Environmental Engineers, Second Edition. Environmental & Engineering Geoscience. 2002;8:244–245. [Google Scholar]
- Daher N, Ruprecht A, Invernizzi G, De Marco C, Miller-Schulze J, Heo JB, Shafer MM, Schauer JJ, Sioutas C. Chemical Characterization and Source Apportionment of Fine and Coarse Particulate Matter Inside the Refectory of Santa Maria Delle Grazie Church, Home of Leonardo Da Vinci’s “Last Supper”. Environmental Science & Technology. 2011;45:10344–10353. doi: 10.1021/es202736a. [DOI] [PubMed] [Google Scholar]
- Delfino RJ, Staimer N, Tjoa T, Arhami M, Polidori A, Gillen DL, George SC, Shafer MM, Schauer JJ, Sioutas C. Associations of primary and secondary organic aerosols with airway and systemic inflammation in an elderly panel cohort. Epidemiology (Cambridge, Mass) 2010;21:892–902. doi: 10.1097/EDE.0b013e3181f20e6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Staimer N, Tjoa T, Gillen DL, Polidori A, Arhami M, Kleinman MT, Vaziri ND, Longhurst J, Sioutas C. Air pollution exposures and circulating biomarkers of effect in a susceptible population: clues to potential causal component mixtures and mechanisms. Environmental Health Perspectives. 2009;117:1232–1238. doi: 10.1289/ehp.0800194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Staimer N, Tjoa T, Polidori A, Arhami M, Gillen DL, Kleinman MT, Vaziri ND, Longhurst J, Zaldivar F, Sioutas C. Circulating Biomarkers of Inflammation, Antioxidant Activity, and Platelet Activation Are Associated with Primary Combustion Aerosols in Subjects with Coronary Artery Disease. Environmental Health Perspectives. 2008;116:898–906. doi: 10.1289/ehp.11189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty KS, Stone EA, Ulbrich IM, DeCarlo PF, Snyder DC, Schauer JJ, Peltier RE, Weber RJ, Murphy SM, Seinfeld JH, Grover BD, Eatough DJ, Jimenez JL. Apportionment of Primary and Secondary Organic Aerosols in Southern California during the 2005 Study of Organic Aerosols in Riverside (SOAR-1) Environmental Science & Technology. 2008;42:7655–7662. doi: 10.1021/es8008166. [DOI] [PubMed] [Google Scholar]
- Ebelt ST, Wilson WE, Brauer M. Exposure to ambient and nonambient components of particulate matter: a comparison of health effects. Epidemiology (Cambridge, Mass) 2005;16:396–405. doi: 10.1097/01.ede.0000158918.57071.3e. [DOI] [PubMed] [Google Scholar]
- Eisner MD, Anthonisen N, Coultas D, Kuenzli N, Perez-Padilla R, Postma D, Romieu I, Silverman EK, Balmes JR. An Official American Thoracic Society Public Policy Statement: Novel Risk Factors and the Global Burden of Chronic Obstructive Pulmonary Disease. American journal of respiratory and critical care medicine. 2010;182:693–718. doi: 10.1164/rccm.200811-1757ST. [DOI] [PubMed] [Google Scholar]
- Fine PM, Cass GR, Simoneit BRT. Characterization of fine particle emissions from burning church candles. Environmental Science & Technology. 1999;33:2352–2362. [Google Scholar]
- Fine PM, Cass GR, Simoneit BRT. Chemical characterization of fine particle emissions from the wood stove combustion of prevalent United States tree species. Environmental Engineering Science. 2004;21:705–721. [Google Scholar]
- Geller MD, Chang M, Sioutas C, Ostro BD, Lipsett MJ. Indoor/outdoor relationship and chemical composition of fine and coarse particles in the southern California deserts. Atmospheric Environment. 2002;36:1099–1110. [Google Scholar]
- Hasheminassab S, Daher N, Schauer JJ, Sioutas C. Source apportionment and organic compound characterization of ambient ultrafine particulate matter (PM) in the Los Angeles Basin. Atmospheric Environment. 2013;79:529–539. [Google Scholar]
- Heo J, Dulger M, Olson MR, McGinnis JE, Shelton BR, Matsunaga A, Sioutas C, Schauer JJ. Source apportionments of PM2.5 organic carbon using molecular marker Positive Matrix Factorization and comparison of results from different receptor models. Atmospheric Environment. 2013;73:51–61. [Google Scholar]
- Herner JD, Green PG, Kleeman MJ. Measuring the Trace Elemental Composition of Size-Resolved Airborne Particles. Environmental Science & Technology. 2006;40:1925–1933. doi: 10.1021/es052315q. [DOI] [PubMed] [Google Scholar]
- Holguin F. Traffic, Outdoor Air Pollution, and Asthma. Immunology and Allergy Clinics of North America. 2008;28:577–588. doi: 10.1016/j.iac.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Hopke PK, Ramadan Z, Paatero P, Norris GA, Landis MS, Williams RW, Lewis CW. Receptor modeling of ambient and personal exposure samples: 1998 Baltimore Particulate Matter Epidemiology-Exposure Study. Atmospheric Environment. 2003;37:3289–3302. [Google Scholar]
- Hughes LS, Allen JO, Salmon LG, Mayo PR, Johnson RJ, Cass GR. Evolution of nitrogen species air pollutants along trajectories crossing the Los Angeles area. Environmental science & technology. 2002;36:3928–3935. doi: 10.1021/es0110630. [DOI] [PubMed] [Google Scholar]
- Ibanez Y, Bot BL, Glorennec P. House-dust metal content and bioaccessibility: a review. European Journal of Mineralogy. 2012;22:629–637. [Google Scholar]
- Jenkins PL, Phillips TJ, Mulberg EJ, Hui SP. Activity patterns of Californians: Use of and proximity to indoor pollutant sources. Atmospheric Environment. Part A. General Topics. 1992;26:2141–2148. [Google Scholar]
- Jones NC, Thornton CA, Mark D, Harrison RM. Indoor/outdoor relationships of particulate matter in domestic homes with roadside, urban and rural locations. Atmospheric Environment. 2000;34:2603–2612. [Google Scholar]
- Kam W, Liacos JW, Schauer JJ, Delfino RJ, Sioutas C. Size-segregated composition of particulate matter (PM) in major roadways and  surface streets. Atmospheric Environment. 2012;55:90–97. [Google Scholar]
- Kleeman MJ, Robert MA, Riddle SG, Fine PM, Hays MD, Schauer JJ, Hannigan MP. Size distribution of trace organic species emitted from biomass combustion and meat charbroiling. Atmospheric Environment. 2008;42:6152–6154. [Google Scholar]
- Klepeis NE, Nelson WC, Ott WR, Robinson JP, Tsang AM, Switzer P, Behar JV, Hern SC, Engelmann WH. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. Journal of exposure analysis and environmental epidemiology. 2001;11:231–252. doi: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- Koenig JQ, Mar TF, Allen RW, Jansen K, Lumley T, Sullivan JH, Trenga CA, Larson TV, Liu LJS. Pulmonary Effects of Indoor- and Outdoor-Generated Particles in Children with Asthma. Environmental Health Perspectives. 2005;113:499–503. doi: 10.1289/ehp.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koistinen KJ, Edwards RD, Mathys P, Ruuskanen J, Künzli N, Jantunen MJ. Sources of fine particulate matter in personal exposures and residential indoor, residential outdoor and workplace microenvironments in the Helsinki phase of the EXPOLIS study. Scandinavian Journal of Work, Environment & Health. 2004;30:36–46. [PubMed] [Google Scholar]
- Kopperud RJ, Ferro AR, Hildemann LM. Outdoor versus indoor contributions to indoor particulate matter (PM) determined by mass balance methods. Journal of the Air & Waste Management Association (1995) 2004;54:1188–1196. doi: 10.1080/10473289.2004.10470983. [DOI] [PubMed] [Google Scholar]
- Kuhn T, Biswas S, Sioutas C. Diurnal and seasonal characteristics of particle volatility and chemical composition in the vicinity of a light-duty vehicle freeway. Atmospheric Environment. 2005;39:7154–7166. [Google Scholar]
- Larson T, Gould T, Simpson C, Liu LJS, Claiborn C, Lewtas J. Source Apportionment of Indoor, Outdoor, and Personal PM2.5 in Seattle, Washington, Using Positive Matrix Factorization. Journal of the Air & Waste Management Association. 2004;54:1175–1187. doi: 10.1080/10473289.2004.10470976. [DOI] [PubMed] [Google Scholar]
- Liacos JW, Kam W, Delfino RJ, Schauer JJ, Sioutas C. Characterization of organic, metal and trace element PM2.5 species and derivation of freeway-based emission rates in Los Angeles, CA. Science of The Total Environment. 2012;435–436:159–166. doi: 10.1016/j.scitotenv.2012.06.106. [DOI] [PubMed] [Google Scholar]
- Liu DL, Nazaroff WW. Particle Penetration Through Building Cracks. Aerosol Science and Technology. 2003;37:565–573. [Google Scholar]
- Liu Y, Zhu L, Shen X. Polycyclic Aromatic Hydrocarbons (PAHs) in Indoor and Outdoor Air of Hangzhou, China. Environmental Science & Technology. 2001;35:840–844. doi: 10.1021/es001354t. [DOI] [PubMed] [Google Scholar]
- Long CM, Suh HH, Catalano PJ, Koutrakis P. Using Time- and Size-Resolved Particulate Data To Quantify Indoor Penetration and Deposition Behavior. Environmental Science & Technology. 2001;35:2089–2099. doi: 10.1021/es001477d. [DOI] [PubMed] [Google Scholar]
- Long CM, Suh HH, Koutrakis P. Characterization of Indoor Particle Sources Using Continuous Mass and Size Monitors. Journal of the Air & Waste Management Association. 2000;50:1236–1250. doi: 10.1080/10473289.2000.10464154. [DOI] [PubMed] [Google Scholar]
- Lough GC, Christensen CG, Schauer JJ, Tortorelli J, Mani E, Lawson DR, Clark NN, Gabele PA. Development of Molecular Marker Source Profiles for Emissions from On-Road Gasoline and Diesel Vehicle Fleets. Journal of the Air & Waste Management Association. 2007;57:1190–1199. doi: 10.3155/1047-3289.57.10.1190. [DOI] [PubMed] [Google Scholar]
- Lunden MM, Thatcher TL, Hering SV, Brown NJ. Use of Time- and Chemically Resolved Particulate Data To Characterize the Infiltration of Outdoor PM2.5 into a Residence in the San Joaquin Valley. Environmental Science & Technology. 2003;37:4724–4732. doi: 10.1021/es026387i. [DOI] [PubMed] [Google Scholar]
- Mader BT, Pankow JF. Study of the Effects of Particle-Phase Carbon on the Gas/Particle Partitioning of Semivolatile Organic Compounds in the Atmosphere Using Controlled Field Experiments. Environmental Science & Technology. 2002;36:5218–5228. doi: 10.1021/es011048v. [DOI] [PubMed] [Google Scholar]
- Manchester-Neesvig JB, Schauer JJ, Cass GR. The Distribution of Particle-Phase Organic Compounds in the Atmosphere and Their Use for Source Apportionment during the Southern California Children’s Health Study. Journal of the Air & Waste Management Association. 2003;53:1065–1079. doi: 10.1080/10473289.2003.10466265. [DOI] [PubMed] [Google Scholar]
- Meng QY, Turpin BJ, Lee JH, Polidori A, Weisel CP, Morandi M, Colome S, Zhang J, Stock T, Winer A. How Does Infiltration Behavior Modify the Composition of Ambient PM2.5 in Indoor Spaces? An Analysis of RIOPA Data. Environmental Science & Technology. 2007;41:7315–7321. doi: 10.1021/es070037k. [DOI] [PubMed] [Google Scholar]
- Miet K, Le Menach K, Flaud PM, Budzinski H, Villenave E. Heterogeneous reactivity of pyrene and 1-nitropyrene with NO2: Kinetics, product yields and mechanism. Atmospheric Environment. 2009;43:837–843. [Google Scholar]
- Minguillón MC, Schembari A, Triguero-Mas M, de Nazelle A, Dadvand P, Figueras F, Salvado JA, Grimalt JO, Nieuwenhuijsen M, Querol X. Source apportionment of indoor, outdoor and personal PM2.5 exposure of pregnant women in Barcelona, Spain. Atmospheric Environment. 2012;59:426–436. [Google Scholar]
- Misra C, Singh M, Shen S, Sioutas C, Hall PM. Development and evaluation of a personal cascade impactor sampler (PCIS) Journal of Aerosol Science. 2002;33:1027–1047. [Google Scholar]
- Naik DV, Weschler CJ, Shields HC. Indoor and outdoor concentrations of organic compounds associated with airborne particles: Results using a novel solvent system. In: Kay JG, Keller GE, Miller JF, editors. Indoor Air Pollution: Radon, Bioaerosols & VOCs. Lewis Publishers; MI: 1991. p. 67. [Google Scholar]
- Nicolaides N. Skin Lipids: Their Biochemical Uniqueness. Science. 1974;186:19–26. doi: 10.1126/science.186.4158.19. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuijsen MJ, Dadvand P, Grellier J, Martinez D, Vrijheid M. Environmental risk factors of pregnancy outcomes: a summary of recent meta-analyses of epidemiological studies. Environmental Health. 2013:12. doi: 10.1186/1476-069X-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntziachristos L, Ning Z, Geller MD, Sheesley RJ, Schauer JJ, Sioutas C. Fine, ultrafine and nanoparticle trace element compositions near a major freeway with a high heavy-duty diesel fraction. Atmospheric Environment. 2007a;41:5684–5696. [Google Scholar]
- Ntziachristos L, Ning Z, Geller MD, Sioutas C. Particle Concentration and Characteristics near a Major Freeway with Heavy-Duty Diesel Traffic. Environmental Science & Technology. 2007b;41:2223–2230. doi: 10.1021/es062590s. [DOI] [PubMed] [Google Scholar]
- Oeder S, Dietrich S, Weichenmeier I, Schober W, Pusch G, Jörres RA, Schierl R, Nowak D, Fromme H, Behrendt H, Buters JTM. Toxicity and elemental composition of particulate matter from outdoor and indoor air of elementary schools in Munich, Germany. Indoor Air. 2012;22:148–158. doi: 10.1111/j.1600-0668.2011.00743.x. [DOI] [PubMed] [Google Scholar]
- Ogulei D, Hopke PK, Wallace LA. Analysis of indoor particle size distributions in an occupied townhouse using positive matrix factorization. Indoor Air. 2006;16:204–215. doi: 10.1111/j.1600-0668.2006.00418.x. [DOI] [PubMed] [Google Scholar]
- Ohura T, Amagai T, Sugiyama T, Fusaya M, Matsushita H. Characteristics of particle matter and associated polycyclic aromatic hydrocarbons in indoor and outdoor air in two cities in Shizuoka, Japan. Atmospheric Environment. 2004;38:2045–2054. [Google Scholar]
- Ohura T, Sugiyama T, Amagai T, Fusaya M, Matsushita H. Simultaneous Liquid Chromatographic Determination of 39 Polycyclic Aromatic Hydrocarbons in Indoor and Outdoor Air and Application to a Survey on Indoor Air Pollution in Fuji, Japan. Journal of AOAC International. 2002;85:188–202. [PubMed] [Google Scholar]
- Olson DA, Turlington J, Duvall RM, McDow SR, Stevens CD, Williams R. Indoor and outdoor concentrations of organic and inorganic molecular markers: Source apportionment of PM2.5 using low-volume samples. Atmospheric Environment. 2008;42:1742–1751. [Google Scholar]
- Orasche Jr, Seidel T, Hartmann H, Schnelle-Kreis Jr, Chow JC, Ruppert H, Zimmermann R. Comparison of Emissions from Wood Combustion. Part 1: Emission Factors and Characteristics from Different Small-Scale Residential Heating Appliances Considering Particulate Matter and Polycyclic Aromatic Hydrocarbon (PAH)-Related Toxicological Potential of Particle-Bound Organic Species. Energy & Fuels. 2012;26:6695–6704. [Google Scholar]
- Phuleria HC, Sheesley RJ, Schauer JJ, Fine PM, Sioutas C. Roadside measurements of size-segregated particulate organic compounds near gasoline and diesel-dominated freeways in Los Angeles, CA. Atmospheric Environment. 2007;41:4653–4671. [Google Scholar]
- Polidori A, Arhami M, Sioutas C, Delfino RJ, Allen R. Indoor/Outdoor Relationships, Trends, and Carbonaceous Content of Fine Particulate Matter in Retirement Homes of the Los Angeles Basin. Journal of the Air & Waste Management Association. 2007;57:366–379. doi: 10.1080/10473289.2007.10465339. [DOI] [PubMed] [Google Scholar]
- Polidori A, Cheung KL, Arhami M, Delfino RJ, Schauer JJ, Sioutas C. Relationships between size-fractionated indoor and outdoor trace elements at four retirement communities in southern California. Atmos Chem Phys. 2009;9:4521–4536. [Google Scholar]
- Polidori A, Turpin B, Meng QY, Lee JH, Weisel C, Morandi M, Colome S, Stock T, Winer A, Zhang J, Kwon J, Alimokhtari S, Shendell D, Jones J, Farrar C, Maberti S. Fine organic particulate matter dominates indoor-generated PM2.5 in RIOPA homes. Journal of Exposure Science and Environmental Epidemiology. 2006;16:321–331. doi: 10.1038/sj.jes.7500476. [DOI] [PubMed] [Google Scholar]
- Rim D, Wallace L, Persily A. Infiltration of Outdoor Ultrafine Particles into a Test House. Environmental Science & Technology. 2010;44:5908–5913. doi: 10.1021/es101202a. [DOI] [PubMed] [Google Scholar]
- Rogge WF, Hildemann LM, Mazurek MA, Cass GR, Simoneit BRT. Sources of fine organic aerosol. 4. Particulate abrasion products from leaf surfaces of urban plants. Environmental Science & Technology. 1993;27:2700–2711. [Google Scholar]
- Rogge WF, Hildemann LM, Mazurek MA, Cass GR, Simoneit BRT. Sources of fine organic aerosol. 8. Boilers burning No. 2 distillate fuel oil. Environmental science & technology. 1997;31:2731–2737. doi: 10.1021/es00056a030. [DOI] [PubMed] [Google Scholar]
- Ruehl CR, Ham WA, Kleeman MJ. Temperature-induced volatility of molecular markers in ambient airborne particulate matter. Atmos Chem Phys. 2011;11:67–76. [Google Scholar]
- Sannigrahi P, Sullivan AP, Weber RJ, Ingall ED. Characterization of water-soluble organic carbon in urban atmospheric aerosols using solid-state 13C NMR spectroscopy. Environmental science & technology. 2006;40:666–672. doi: 10.1021/es051150i. [DOI] [PubMed] [Google Scholar]
- Sardar SB, Fine PM, Sioutas C. Seasonal and spatial variability of the size-resolved chemical composition of particulate matter (PM10) in the Los Angeles Basin. Journal of Geophysical Research: Atmospheres. 2005;110 (D7):D07S08. [Google Scholar]
- Sarnat SE, Coull BA, Ruiz PA, Koutrakis P, Suh HH. The Influences of Ambient Particle Composition and Size on Particle Infiltration in Los Angeles, CA, Residences. Journal of the Air & Waste Management Association. 2006;56:186–196. doi: 10.1080/10473289.2006.10464449. [DOI] [PubMed] [Google Scholar]
- Sarwar G, Olson DA, Corsi RL, Weschler CJ. Indoor fine particles: the role of terpene emissions from consumer products. Journal of the Air & Waste Management Association (1995) 2004;54:367–377. doi: 10.1080/10473289.2004.10470910. [DOI] [PubMed] [Google Scholar]
- Schauer JJ. Environmental Engineering Science. California Institute of Technology; Pasadena: 1998. Souce contributions to atmospheric organic compound concentrations: emission measurements and model predicitons; p. 400. [Google Scholar]
- Schauer JJ. Evaluation of elemental carbon as a marker for diesel particulate matter. Journal of Exposure Science and Environmental Epidemiology. 2003;13:443–453. doi: 10.1038/sj.jea.7500298. [DOI] [PubMed] [Google Scholar]
- Schauer JJ, Kleeman MJ, Cass GR, Simoneit BRT. Measurement of emissions from air pollution sources. 1. C-1 through C-29 organic compounds from meat charbroiling. Environmental Science & Technology. 1999;33:1566–1577. doi: 10.1021/es001331e. [DOI] [PubMed] [Google Scholar]
- Schauer JJ, Rogge WF, Hildemann LM, Mazurek MA, Cass GR, Simoneit BRT. Source apportionment of airborne particulate matter using organic compounds as tracers. Atmospheric Environment. 1996;30:3837–3855. [Google Scholar]
- Sheesley RJ, Schauer JJ, Zheng M, Wang B. Sensitivity of molecular marker-based CMB models to biomass burning source profiles. Atmospheric Environment. 2007;41:9050–9063. [Google Scholar]
- Shi T, Schins RPF, Knaapen AM, Kuhlbusch T, Pitz M, Heinrich J, Borm PJA. Hydroxyl radical generation by electron paramagnetic resonance as a new method to monitor ambient particulate matter composition. Journal of Environmental Monitoring. 2003;5:550–556. doi: 10.1039/b303928p. [DOI] [PubMed] [Google Scholar]
- Simoneit BRT. Characterization of Organic Constituents in Aerosols in Relation to Their rigin and Transport: A Review. International Journal of Environmental Analytical Chemistry. 1986;23:207–237. [Google Scholar]
- Singer BC, Coleman BK, Destaillats H, Hodgson AT, Lunden MM, Weschler CJ, Nazaroff WW. Indoor secondary pollutants from cleaning product and air freshener use in the presence of ozone. Atmospheric Environment. 2006;40:6696–6710. doi: 10.1021/es052198z. [DOI] [PubMed] [Google Scholar]
- Singh M, Jaques PA, Sioutas C. Size distribution and diurnal characteristics of particle-bound metals in source and receptor sites of the Los Angeles Basin. Atmospheric Environment. 2002;36:1675–1689. [Google Scholar]
- Snyder DC, Rutter AP, Collins R, Worley C, Schauer JJ. Insights into the Origin of Water Soluble Organic Carbon in Atmospheric Fine Particulate Matter. Aerosol Science and Technology. 2009;43:1099–1107. [Google Scholar]
- Stone EA, Snyder DC, Sheesley RJ, Sullivan AP, Weber RJ, Schauer JJ. Source apportionment of fine organic aerosol in Mexico City during the MILAGRO experiment 2006. Atmos Chem Phys. 2008;8:1249–1259. [Google Scholar]
- Suh HH, Koutrakis P, Spengler JD. The relationship between airborne acidity and ammonia in indoor environments. Journal of exposure analysis and environmental epidemiology. 1994;4:1–22. [PubMed] [Google Scholar]
- Szymczak W, Menzel N, Keck L. Emission of ultrafine copper particles by universal motors controlled by phase angle modulation. Journal of Aerosol Science. 2007;38:520–531. [Google Scholar]
- Thatcher TL, Layton DW. Deposition, resuspension, and penetration of particles within a residence. Atmospheric Environment. 1995;29:1487–1497. [Google Scholar]
- Turpin BJ, Lim HJ. Species contributions to PM2. 5 mass concentrations: Revisiting common assumptions for estimating organic mass. Aerosol Science & Technology. 2001;35:602–610. [Google Scholar]
- Valavanidis A, Fiotakis K, Bakeas E, Vlahogianni T. Electron paramagnetic resonance study of the generation of reactive oxygen species catalysed by transition metals and quinoid redox cycling by inhalable ambient particulate matter. Redox Report. 2005;10:37–51. doi: 10.1179/135100005X21606. [DOI] [PubMed] [Google Scholar]
- Watson JG, Cooper JA, Huntzicker JJ. The effective variance weighting for least squares calculations applied to the mass balance receptor model. Atmospheric Environment (1967) 1984;18:1347–1355. [Google Scholar]
- Watson JG, Robinson NF, Lewis C, Coulter T. Chemical Mass Balance Receptor Model Version 8 (CMB8) User’s Manual. Desert Research Institute; Reno, NV: 1997. Document No. 1808.1D1. [Google Scholar]
- Weber RJ, Sullivan AP, Peltier RE, Russell A, Yan B, Zheng M, de Gouw J, Warneke C, Brock C, Holloway JS, Atlas EL, Edgerton E. A study of secondary organic aerosol formation in the anthropogenic-influenced southeastern United States. Journal of Geophysical Research: Atmospheres. 2007;112(D13):D13302. [Google Scholar]
- Weschler CJ. Chemical reactions among indoor pollutants: what we’ve learned in the new millennium. Indoor Air. 2004;14:184–194. doi: 10.1111/j.1600-0668.2004.00287.x. [DOI] [PubMed] [Google Scholar]
- Weschler CJ, Shields HC. Potential reactions among indoor pollutants. Atmospheric Environment. 1997;31:3487–3495. [Google Scholar]
- Zhang Y, Schauer JJ, Shafer MM, Hannigan MP, Dutton SJ. Source Apportionment of in Vitro Reactive Oxygen Species Bioassay Activity from Atmospheric Particulate Matter. Environmental Science & Technology. 2008;42:7502–7509. doi: 10.1021/es800126y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.