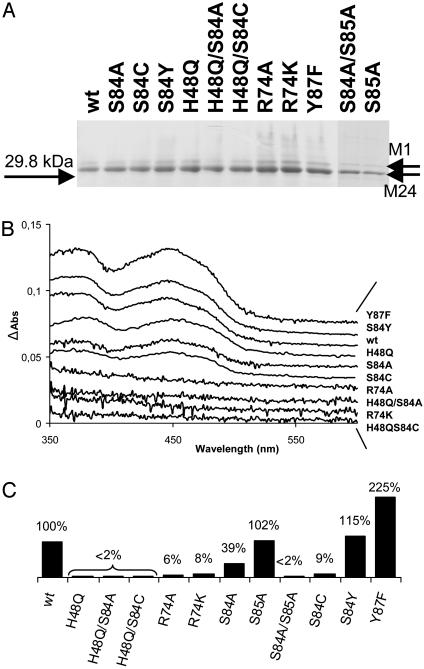
Fig. 3.
(A) SDS/10% PAGE of H. pylori wild-type (wt) and mutated ThyX proteins by using ≈1 μg of purified protein stained with Coomassie Brilliant Blue. M1 and M24 refer to the alternative start codons of H. pylori HP1533. (B) Spectroscopic analysis of H. pylori ThyX wild-type and mutant proteins (1). (C) “Deprotonation” activity of H. pylori ThyX proteins with 6.25 μM dUMP at 37°C as described in Materials and Methods. Complete reaction mixtures contained 50 mM Hepes (pH 7.0), 10% glycerol, 0.9 mM CH2H4folate, 10 mM MgCl2, 2 mM NADPH, 1 mM NADH, 0.5 mM FAD, and 6.25 μM [5-3H]dUMP (specific activity, 2.55 Ci/mmol). The specific activity for the wild-type protein corresponding to 0.95 nmol of 3H2O formed per min per mg of protein was arbitrarily chosen to correspond to 100%.