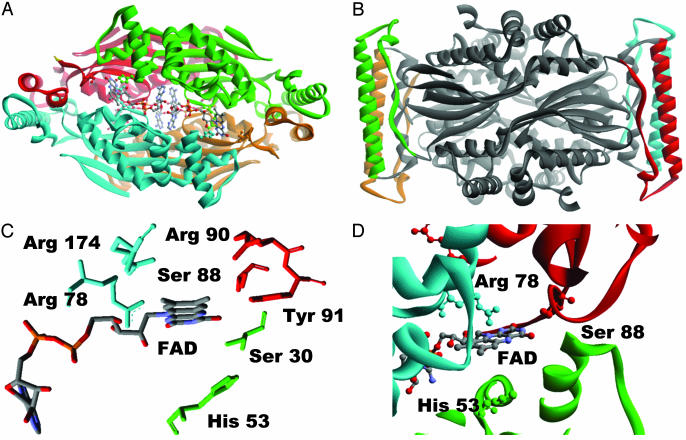
Fig. 5.
(A) ThyX tetramer (4) where the different subunits are shown with the different colors. FAD, S88, H53, and R78 (corresponding to active-site residues of H. pylori ThyX identified in this work) are represented with balls and sticks. (B) The central variable part of the ThyX protein indicated with the green dotted line in Fig. 1 is indicated by the different colors by monomer. (C) Only the conserved residues located within 7 Å of the redox-active N5 atom of the FAD molecule (only one of four FAD molecules of homotetramer is shown) are represented (see also Fig. 1). (D) The active-site configuration of T. maritima ThyX protein. A FAD molecule, Arg-78, Ser-88, and His-53 (T. maritima ThyX numbering) corresponding, respectively, to Ser-84, His-48, and Arg-74 in H. pylori protein are represented by balls and sticks. The different subunits of ThyX homotetramer are shown with the different colors. Note that the residues from the three different subunits participate in the formation of the single active site.