Table 1. Genetic and biochemical characterization of H. pylori ThyX mutant proteins.
| Kinetic parameters* deprotonation activity
|
||||||
|---|---|---|---|---|---|---|
| Complementation activity
|
FAD binding, %
|
kcat/Km
|
||||
| H. pylori thyX allele |
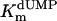 , μM , μM |
kcat, min−1 | min−1·μM−1 | %† | ||
| Wild-type | +++ | 100 | 7.2 ± 2.52 | 0.46 ± 0.02 | 0.064 | 100 |
| H48Q | − | 77.9 | ND | ND | ND | ND |
| H48Q/S84A | − | 10.3 | ND | ND | ND | ND |
| H48Q/S84C | − | 5.9 | ND | ND | ND | ND |
| R74A | − | 12.2 | 37.0 | 0.10 | 0.003 | 4.7 |
| R74K | − | 7.7 | 7.8 | 0.07 | 0.009 | 14.1 |
| S84A | − | 56.9 | 38.4 | 0.22 | 0.006 | 9.4 |
| S85A | +++ | 107.8 | NM | NM | NM | NM |
| S84A/S85A | − | 22.8 | ND | ND | ND | ND |
| S84C | − | 53.7 | 14.0 (n = 1) | 0.05 | 0.004 | 6.3 |
| S84Y | +++ | 113.1 | 9.7 (n = 1) | 0.72 | 0.074 | 115.6 |
| Y87F | +++ | 158.3 | 3.8 (n =1) | 0.48 | 0.126 | 196.9 |
Standard deviations shown for Km and kcat values obtained for wild-type protein were calculated by using five different data sets. Unless otherwise indicated, all other values are the averages of two independent measures. ND, nondetectable activity; NM, not measured.
100% refers to kcat/Km value of 0.064 min−1·μM−1 measured for wild-type protein.
