Abstract
Nitric oxide (NO) releasing (NORel) materials have been extensively investigated to create localized increases in NO concentration by the proton driven diazeniumdiolate-containing polymer coatings and demonstrated to improve extracorporeal circulation (ECC) hemocompatibility. In this work, the NORel polymeric coating composed of a diazeniumdiolated dibutylhexanediamine (DBHD-N2O2)-containing hydrophobic Elast-eon™ (E2As) polyurethane was combined with a direct thrombin inhibitor, argatroban (AG), and evaluated in a 4 h rabbit thrombogenicity model without systemic anticoagulation. In addition, the immobilizing of argatroban to E2As polymer was achieved by either a polyethylene glycol-containing (PEGDI) or hexane methylene (HMDI) diisocyanate linker. The combined polymer film was coated on the inner walls of ECC circuits to yield significantly reduced ECC thrombus formation compared to argatroban alone ECC control after 4 h blood exposure (0.6 ± 0.1 AG/HMDI/NORel vs 1.7 ± 0.2 cm2 AG/HMDI control). Platelet count (2.8 ± 0.3 AG/HMDI/NORel vs 1.9 ± 0.1 × 108/ml AG/HMDI control) and plasma fibrinogen levels were preserved after 4 h blood exposure with both the NORel/argatroban combination and the AG/HMDI control group compared to baseline. Platelet function as measured by aggregometry remained near normal in both the AG/HMDI/NORel (63 ± 5%) and AG/HMDI control (58 ± 7%) groups after 3 h compared to baseline (77 ± 1%). Platelet P-selectin mean fluorescence intensity (MFI) as measured by flow cytometry also remained near baseline levels after 4 h on ECC to ex vivo collagen stimulation (16 ± 3 AG/HMDI/NORel vs 11 ± 2 MFI baseline). These results suggest that the combined AG/HMDI/NORel polymer coating preserves platelets in blood exposure to ECCs to a better degree than AG/PEGDI/NORel, NORel alone or AG alone. These combined antithrombin, NO-mediated antiplatelet effects were shown to improve thromboresistance of the AG/HMDI/NORel polymer-coated ECCs and move potential nonthrombogenic polymers closer to mimicking vascular endothelium.
Keywords: argatroban, direct thrombin inhibition, nitric oxide releasing polymer, thrombogenicity, hexane methylene diisocyanate (HMDI), polyethylene glycol (PEG)
1. Introduction
Extracorporeal devices (i.e., cardio-pulmonary bypass, hemodialysis, hemofiltration, extracorporeal membrane oxygenation) require systemic anticoagulation. The major complications are clotting in the device and bleeding from the patient. Systemic anticoagulation blocks fibrin formation but does not prevent platelet adhesion and activation [1, 2]. In an attempt to reduce the activation of platelets at the blood/material surface interface, we and other investigators have developed surface coatings that mimic the vascular endothelium and preserve platelet quiescence in the extracorporeal circuits (ECC). Nitric oxide, an endogenous platelet inhibitor and vasodilator, has been incorporated into polymeric tubings to mimic its endogenous effects on maintaining platelet quiescence. Indeed, a number of NO releasing (NORel) polymer coatings have been studied using polymer-embedded NO donor molecules, such as diazeniumdiolated dibutylhexanediamine (DBHD/N2O2) [3–9]. The NO released from the DBHD/N2O2 polymer coating has proven to effectively maintain circulating platelets in a resting state and reduce thrombus formation in the ECCs even when no systemic anticoagulation is present. However, small fibrin thrombi are present in animal models. An immobilized anticoagulant in combination with the NORel polymer may provide the complete nonthrombogenic scenario needed for ECLS circuit longevity.
Commercial availability of heparin-coated components for ECLS circuits such as catheters and oxygenators has brought immobilized anticoagulants to the forefront for over 20 years. Unfortunately systemic anticoagulation is still required in all clinical ECLS procedures, albeit at a reduced level [2, 10]. The use of heparin-coated ECLS components is dependent upon the patient plasma levels of antithrombin III (ATIII) to which heparin needs to bind before the heparin/ATIII complex directly inhibits any released thrombin. Varying ATIII plasma concentrations could be an important explanation for varying nonthrombogenic effects of the heparin-coated tubing and devices. Therefore, use of a direct thrombin inhibitor such as argatroban would be an effective means to inhibit thrombin activity regardless of the plasma ATIII concentrations.
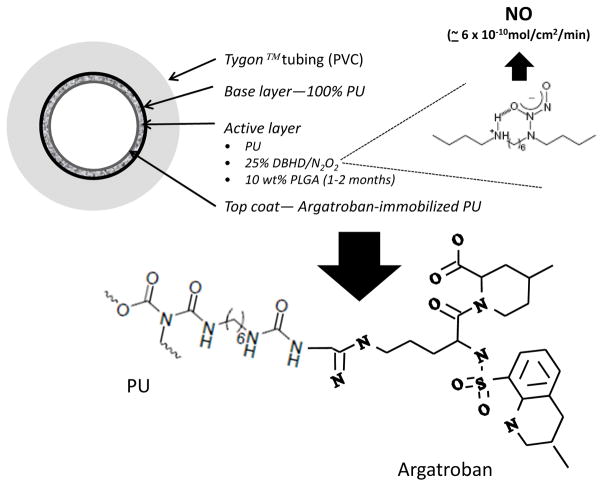
Argatroban, a synthetic small molecule (Figure 1), was developed to provide better nonthrombotic efficacy over heparin [11]. After 2 decades of research, direct thrombin inhibition particularly with argatroban has proven to provide adequate anticoagulation clinically [12]. Studying the effects of the combined argatroban and NORel polymer coatings in blocking both fibrin formation and platelet activation is a logical next step of investigation (Figure 2).
Figure 1.
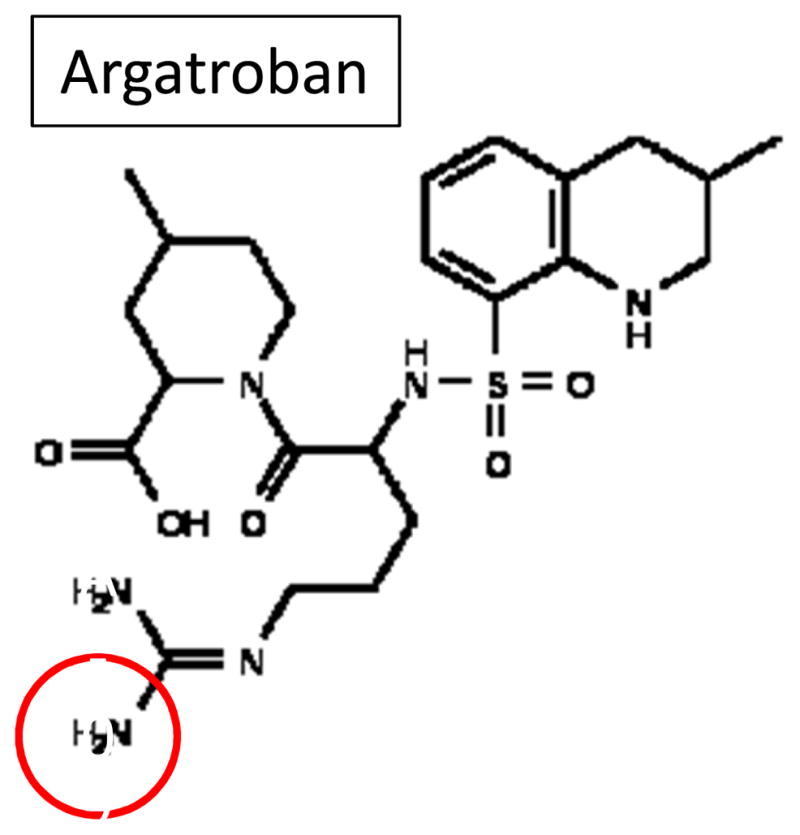
Structure of argatroban ((2R,4R)-1-[(2S)-5-(diaminomethylideneamino)-2-[[(3R)-3-methyl-1,2,3,4-tetrahydroquinolin-8-yl]sulfonylamino]pentanoyl]-4-methyl-piperidine-2-carboxylic acid). Chemically, argatroban (MW= 509) is the dipeptide between arginine and 4-methyl-2-piperidine carboxylic acid with the NH2 closest to the carbonyl group of arginine bonded to a methyltetrahydroquinoline sulfonyl group. The immobilization of argatroban was accomplished by linking the free primary amine of the arginine ‘tail’ to an isocyanate group of either HMDI or PEG-DI (red circle).
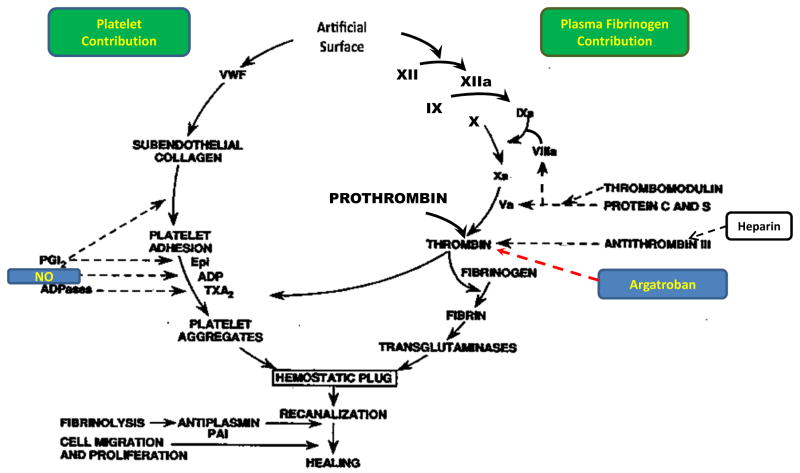
Figure 2.
Overview of hemostasis. Hemostasis for maintaining a thrombotic environment when blood comes into contact with foreign surfaces such as extracorporeal life support (ECLS) circuits consists of two contributions. One is the intrinsic coagulation pathway (right) that initiates with factor XII activation and ending with the formation of a fibrin clot. The second contribution is the activation of blood cell components particularly the circulating platelets (left). Both contribution pathways converge at a point of thrombin formation which forms the beginning of the common thrombotic pathway and ultimate hemostatic plug which consists of the fibrin clot and aggregated platelets. As the hemostatic plug matures other blood cell components such as red blood cells (RBC) and activated white blood cells (WBC) also bind in the plug. Fibrinolysis of the plug in the ECLS circuit may not be as important of a process as it is in vessel wounds and healing (i.e., extrinsic coagulation pathway; not shown). The ‘a’ following the various coagulation factors (right) means activated form of factor. On the left side of hemostasis, vWF is von Willibrand factor, epi= epinephrine, ADP= adenosine diphosphate, TXA2= thromboxane A2, PGI2= prostacyclin and ADPases= adenosine diphosphatases. Two inhibitors of thrombosis are shown on schematic with heparin used to block fibrin formation and endothelial-derived nitric oxide (NO) which inhibits platelet activation.
The present study was designed to evaluate by the NORel and argatroban combination. This combination of the direct thrombin inhibitor, argatroban, and NORel would provide clinicians medical devices for patients who demonstrate the adverse immune-mediated response to administered heparin namely heparin-induced thrombocytopenia (HITS) [13].
2. Materials and Methods
2.1. Materials
Tygon® poly(vinyl chloride) (PVC) tubing was purchased from Fisher Healthcare (Houston, TX). The hydrophobic polyurethane, Elast-Eon (E2As), was a product of AorTech Biomaterials, Inc. (Rogers, MN). Anhydrous tetrahydrofuran (THF), hexamethylene diisocyanate (HMDI) and poly(propylene glycol), tolylene 2, 4-diisocyanate terminated (PEG-DI) were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO). Argatroban monohydrate (((2R,4R)-1-[(2S)-5-(diaminomethylideneamino)-2-[[(3R)-3-methyl-1,2,3,4-tetrahydroquinolin-8-yl]sulfonylamino]pentanoyl]-4-methyl-piperidine-2-carboxylic acid) was also obtained from Sigma-Aldrich (St. Louis, MO). Anhydrous hexanes were purchased from Fisher Scientific Co. (Fair Lawn, NJ). (Z)- 1 -[N-Butyl-N-[6[(N-butylammoniohexl)amino]]-diazen-1-ium-1,2-diolate (diazeniumdiolated dibutylhexanediamine (DBHD/N2O2)) was synthesized by treating N,N0-dibutyl-1,6-hexanediamine (Pflatz & Bauer, Waterbury, CT) with nitric oxide (NO) (80 psi) at room temperature (RT) for 17–24 h as previously described [14]. The mouse antibodies for human CD61 (GPIIIa) FITC and human P-selectin glycoprotein (CD62P) PE were from AbD Serotec (Raleigh, NC) as well as Mouse isotype controls for IgG1 FITC and IgG1 PE. For monocyte surface receptor determination of the integrin, CD11b, and the surface antigen CD14, the rat antibody for mouse CD11b Alexa Flour 488 and mouse antibody for human CD 14 PE, respectively, from AbD Serotec were used. The antibody clones for P-selectin, CD11b and CD14 have been previously shown to have cross-reactivity to the rabbit [15]. The rat isotype control for IgG2b Alexa Fluor 488 and the mouse isotype control for IgG2a PE were purchased from AbD Serotec.
2.2. Synthesis of argatroban polymeric coatings
The E2As polymer, 2 g, was dissolved in 25 ml anhydrous THF in a 100 ml round-bottomed flask and then 4 ml of either HMDI (about 23.8 mmoles) or PEG-DI (number average for degree of polymerization = about 36 mer) was slowly added. The flask was stoppered and the mixture was magnetically stirred for 2 h at room temperature (RT). The reaction product was precipitated and washed several times with anhydrous hexane in a filter paper-covered Buchner funnel (Whatman #5 filter paper, Buckinghamshire, UK) to remove free HMDI or PEG-DI. The filtered intermediate product was air dried for 5 minutes and then dissolved with 25 ml THF in a 100 ml round bottomed flask under stirring. A 10 μl aliquot of this solution was then submitted to Fourier transform infrared spectrometer (Nicolet 6700, Thermo Scientific, Madison, WI; FTIR) to determine incorporation of either HMDI or PEG-DI linker into the E2As polymer backbone. The intermediate product solution was stirred and then drop wise treated with 30 μmoles argatroban, which was dissolved in dimethyl sulfoxide (DMSO) at 100 μmoles/ml, for 24 h at RT. After the reaction, the product was precipitated with deionized water and extensively washed with water in the filter paper-covered Buchner funnel to remove excess argatroban. The product was air dried for 5 minutes under vacuum. The resultant argatroban/E2As linked polymer was dissolved in 25 ml THF and a 10 μl aliquot of this solution was then submitted to FTIR to determine incorporation of argatroban to either HMDI or PEG-DI linker within the E2As polymer backbone. The reactions are illustrated in Figure 3.
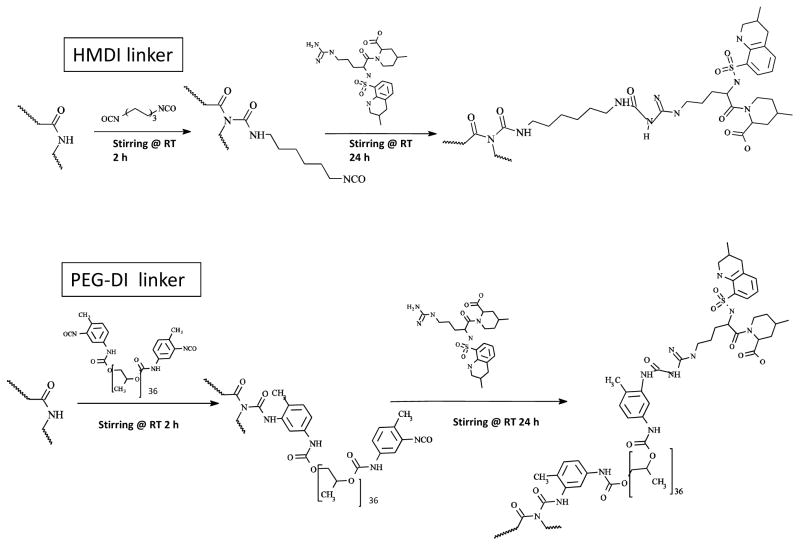
Figure 3.
Chemical synthesis of immobilized argatroban using either HMDI or PEG-DI linkers. Schematic of two-step reaction to produce either HMDI or PEG-DI-linked E2As intermediate polymer and then grafting argatroban onto this intermediate. NCO= isocyanate group and RT= room temperature.
2.3. In vitro antithrombin assay
Films of the argatroban-bound E2As polymer was formed in 96-well plates by adding 100 μl of the modified polymer solution to each well and allowing the THF solvent to evaporate slowly at RT by partially covering plate with its lid. After 24 h, plates were stored at 4°C until assay was run. In order to determine argatroban levels in the modified E2As polymer, the antithrombin activity of the films were compared to the antithrombin activity of a free argatroban standard curve. The assay utilizes a chromogenic substrate which mimics fibrinogen, the natural substrate for the serine protease, thrombin [16]. Briefly, the chromogenic substrate (i.e., N-p-tosyl-Gly-Pro-Arg-p-nitroanilide acetate) (Sigma-Aldrich, St. Louis, MO) is cleaved by thrombin (BC thrombin, Siemens, Newark, DE) into a residual peptide (i.e., Tosyl-Gly-Pro-Arg-OH) and free p-nitroanilide, which is measured at 405 nm in a Biotek Cytation 3 microplate reader (Winooski, VT). The absorbance difference is used for the determination of thrombin inhibition in the assay.
2.4. Preparation of argatroban/NORel polymeric coatings and ECC construction
A polymer coating solution containing 25 wt% DBHD/N2O2 and poly(lactic-co-glycolic acid) (PLGA, 1–2 months) in E2As polyurethane was prepared using a method previously reported [17]. Briefly, double layers of polymeric coatings which included a base layer and an active layer were individually coated on the inner wall of the Tygon® tubing that formed the ECC. The base layer was formulated using 4 wt% of E2As in THF (Soln A). The active layer was prepared by dissolving 4 wt% E2As in THF along with 25 wt% DBHD/N2O2 and 10 wt% of PLGA (Soln B). The top layer was formulated using 4 wt% of the 30 μmoles argatroban/E2As polymer (as described above) in THF (Soln C).
The ECC used in the rabbit model consisted of a 16-gauge and 14-gauge IV polyurethane angiocatheters (Kendall Monoject Tyco Healthcare Mansfield, MA), two 16 cm lengths of ¼ inch inner diameter (ID) Tygon® tubing and a 8 cm length of 3/8 inch ID Tygon® tubing which created a thrombogenicity chamber where thrombus could form more easily due to more turbulent blood flow. The ECC was pieced together, starting at the left carotid artery side, with the 16-gauge angiocatheter, one 15 cm length ¼ inch ID tubing, the 8 cm length thrombogenicity chamber, the second 15 cm length ¼ inch ID tubing and finally the 14-gauge angiocatheter. The angiocatheters were interfaced with tubing using 2 luer-lock PVC connectors. The 3/8 inch ID tubing and the ¼ inch tubing were welded together using THF. Blood flow was continued until the ECC was occluded by clot, or 4 h. No systemic anticoagulation was given.
The assembled ECC was coated first with a base coat of Soln A, followed by two active coats of Soln B and then one top coat of Soln C. The circuitry was filled with each solution then removed. Each coat was allowed to dry for at least 1 h. The finished ECCs were allowed to cure at RT for 2 d, and then stored at 4°C until used (Figure 4).
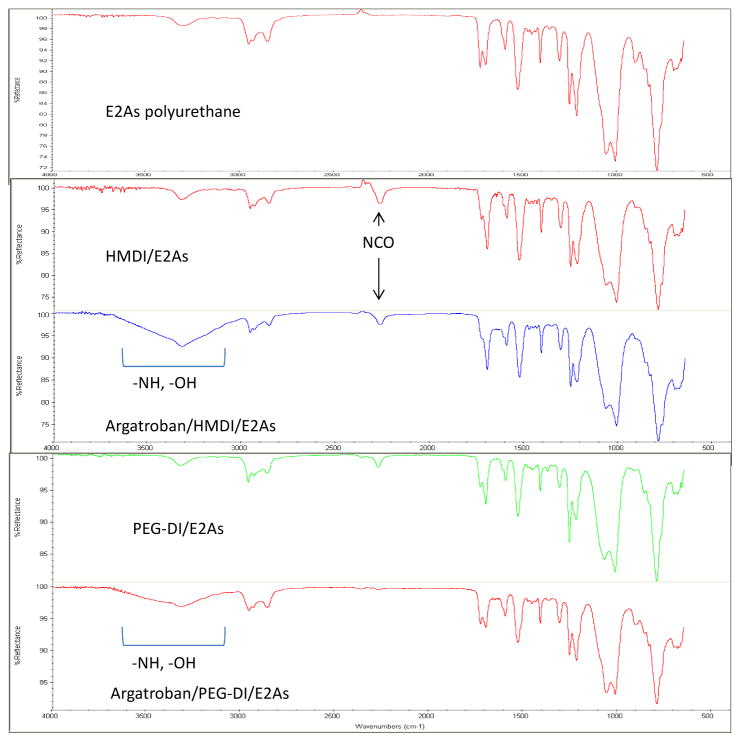
Figure 4.
Fourier transformed infrared spectroscopy (FTIR) spectrum of unmodified E2As polymer (top red), HMDI- (second red) or PEG-DI-linked E2As intermediate (green) and argatroban/HMDI (blue) or PEG-DI/E2As (purple). Broad peak between 3000–3600 cm−1 incorporates most of the secondary and tertiary amines of argatroban while the peak near 2250 cm−1 shows the reduction of peak following grafting of argatroban to HMDI- or PEG-DI-linked intermediates.
2.5. NO release measurements
NO released from the NORel polymer-coated circuits was measured via a Sievers chemiluminescence NO Analyzer® (NOA), model 280 (Boulder, CO) and previously described [3]. Briefly, after a 5 min measurement of baseline NO level, a centimeter square sample of the DBHD/N2O2-containing circuitry was then placed in the reaction vessel containing the substrate solution at 37° C. NO was continuously swept from the headspace of the sample vessel and purged from the bathing solution with a nitrogen sweep gas and bubbler into the chemiluminescence detection chamber. The flow rate was set to 200 ml/min with a chamber pressure of 5.4 Torr and an oxygen pressure of 6.0 psi. Using this method, a uniform segment of NORel polymer was tested for NO release prior to and 4 h after blood exposure.
2.6. The rabbit thrombogenicity model
The animal handling and surgical procedures were approved by the University Committee on the Use and Care of Animals in accordance with university and federal regulations. A total of 32 New Zealand white rabbits (Covance, Battle Creek, MI) were used in this study. All rabbits (2.5–3.5 kg) were initially anesthetized with intramuscular injections of 5 mg/kg xylazine injectable (AnaSed® Lloyd Laboratories Shenandoah, Iowa) and 30 mg/kg ketamine hydrochloride (Hospira, Inc. Lake Forest, IL). Maintenance anesthesia was administered via a diluted intravenous (IV) infusion of ketamine at a rate of 15 mg/kg/h. In order to maintain blood pressure stability, IV fluids of Lactated Ringer’s were given at a rate of 33 ml/kg/h. The paralytic, pancuronium bromide (0.2 mg/kg, IV), was administered to have animal totally dependent upon mechanical ventilation which was done via a tracheotomy and using a Sechrist Infant Ventilator Model IV-100 (Anaheim, CA). For monitoring blood pressure and collecting blood samples, the rabbits’ right carotid artery was cannulated using a 16-gauge IV angiocatheter (Jelco®, Johnson & Johnson, Cincinnati, OH). Blood pressure and derived heart rate were monitored with a Series 7000 Monitor (Marquette Electronics Milwaukee, WI). Body temperature was monitored with a rectal probe and maintained at 37°C using a water-jacketed heating blanket. Prior to placement of the arteriovenous (AV) custom-built extracorporeal circuit (ECC), the rabbit left carotid artery and right external jugular vein were isolated and baseline hemodynamics as well as arterial blood pH, pCO2, pO2, total hemoglobin and methemoglobin were measured using an ABL 725 blood-gas analyzer and an OSM3 Hemoximeter (Radiometer Copenhagen Copenhagen, DK). In addition, baseline blood samples were collected for platelet and total white blood cell (WBC) counts which were measured on a Coulter Counter Z1 (Coulter Electronics Hialeah, FL). Plasma fibrinogen levels were determined using a Dade Behring BCS Coagulation Analyzer (Siemans Deerfield, IL), activated clotting times (ACT) were monitored using a Hemochron Blood Coagulation System Model 801 (International Technidyne Corp. Edison, NJ), platelet function was assessed using a Chrono-Log optical aggregometer model 490 (Havertown, PA) and platelet P-selectin and monocyte CD11b expression were determined utilizing fluorescent-activated cell sorting (FACS) with a Becton Dickinson FACSCalibur flow cytometer (San Jose, CA).
After baseline blood measurements, the AV custom-built ECC was placed into position by cannulating the left carotid artery for ECC inflow and the right external jugular vein for ECC outflow. The flow through the ECC was started by unclamping the arterial and venous sides of ECC and blood flow in circuit was monitored with an ultrasonic flow probe and flow meter (Transonic HT207 Ithaca, NY). Animals were not systemically anticoagulated during the experiments.
After four hours on ECC, the circuits were clamped, removed from animal, rinsed with 60 ml of saline and drained. Any residual thrombus in the larger tubing of ECC (i.e., thrombogenicity chamber) was photographed and the degree of thrombus image was quantitated using Image J imaging software from National Institutes of Health (Bethesda, MD). Prior to euthanasia, all animals received a dose of 400 U/kg sodium heparin to prevent necrotic thrombosis. The animals were euthanized using a dose of Fatal Plus (130 mg/kg sodium pentobarbital) (Vortech Pharmaceuticals Dearborn, MI). All animals underwent gross necropsy after being euthanized, including examination of the lungs, heart, liver and spleen for any signs of thromboembolic events.
2.7. Blood sampling
Rabbit whole blood samples were collected in non-anticoagulated 1 cc syringes for ACT, 10% anticoagulant containing 3.2% sodium citrate in 3 cc Vacutainer® tubes (Becton Dickinson, Franklin Lakes, NJ) for cell counts, aggregometry and FACS analysis, and 1 cc syringes containing 40 U/ml of sodium heparin (APP Pharmaceuticals, LLC Schaumburg, IL) for blood-gas analysis. Following the initiation of ECC blood flow, blood samples were collected every hour for 4 h for in vitro measurements. Samples were used within 2 h of collection to avoid any activation of platelets, monocytes or plasma fibrinogen.
2.8. Platelet aggregometry
Rabbit platelet aggregation was assayed based on the Born’s turbidimetric method using a Chrono-Log optical aggregometer as previously described [1]. Briefly, citrated blood (1:10 blood to ACD) was collected (6 ml) and platelet-rich plasma (PRP) was obtained by centrifugation at 110 × g for 15 min. Platelet-poor plasma (PPP) was obtained by another centrifugation of the PRP-removed blood sample at 2730 × g for 15 min and was used as the blank for aggregation. PRP was incubated for 10 min at 37°C and then 40 μg/ml collagen (Chrono-PAR #385 Havertown, PA) was added. The percentage of aggregation was determined 3 min after the addition of collagen using Chrono-Log Aggrolink software.
2.9. Flow cytometry
To determine platelet P-selectin (CD62P) and CD61 (GPIIIa, beta subunit of fibrinogen receptor) expression, 100 ul of diluted blood aliquots (1:100 dilution of blood to Hank’s Balanced Salt Solution (HBSS) without CaCl2 and MgCl2) were directly prepared for cell surface staining of P-selectin and GPIIIa. In four 12 × 75 polypropylene tubes containing 100 μl of diluted blood, 40 μg/ml collagen (4 μl 1000 μg/ml) was added to two tubes and 4 μl saline was added to the other two tubes. At this point, saturating concentrations (10 μl) of monoclonal antihuman IIIa FITC and monoclonal antihuman CD62P PE antibodies were added to one of the collagen and one of the saline treated tubes and incubated for 15 min at room temperature (RT) in the dark. In the other two tubes containing collagen and saline, 10 μl each of antimouse IgG1 FITC and PE were added as nonbinding isotype controls and also incubated for 15 min at RT in the dark. After the antibody incubation step, each tube received 700 μl of freshly prepared 1% formaldehyde buffer (in dPBS) and was stored at 4°C until ready for flow cytometric analysis. To determine monocyte CD11b and CD14 expression, 100 μl of the undiluted blood aliquots were directly prepared for cell surface staining of CD11b and CD14. At this point, saturating concentrations (10 μl) of rat anti-mouse CD11b Alexa Fluor 488 and monoclonal anti-human CD14 PE antibodies were added to one tube and 10 μl each of anti-rat IgG2b Alexa Fluor 488 and anti-mouse IgG2a PE were added as nonbinding isotype controls. All tubes were incubated for 30 min at 4°C in the dark. After the antibody incubation, lysing of red blood cells was accomplished by adding 2 ml of FACSLysing Buffer (Becton Dickinson San Jose, CA), gentle vortexing and then incubating for 10 min at room temperature in the dark. After red blood cell lysing, centrifugation at 250 × g for 5 min at 4°C pelleted the stained leukocytes. After aspirating supernatant, one wash step was done with wash buffer containing dPBS, 0.1% sodium azide and 0.5% bovine serum albumin, and then the the sample was centrifuged again and the resulting supernatant was aspirated. The cells were then resuspended in 250 ul of freshly prepared 1% formaldehyde buffer and stored at 4°C until ready for flow cytometric analysis. A FACSCalibur flow cytometer (Becton Dickinson San Jose, CA) was used for the acquisition of flow data and the CellQuest software was employed for data analysis. Cell populations were identified for data collection by their forward scatter (FSC) and side scatter (SSC) light profiles. For each sample, 30,000 total events were collected. Fluorescence intensity of immunostaining was quantitated by a histogram log plot analysis. Mean fluorescent intensity (MFI) was expressed as the geometric mean channel fluorescence minus the appropriate isotype control.
Statistical Analysis
Data are expressed as mean ± SEM (standard error of the mean). Comparison between the argatroban/NORel, argatroban and NORel controls and E2As control polymer groups were analyzed by the one-way ANOVA with a multiple comparison of means using a nonparametric Wilcoxon/Kruskall Wallis rank sum test. All statistical analyses were performed using the statistical program SAS JMP (SAS Institute Cary, NC). Nonparametric analysis was used due to the lack of normal distribution of sample variances.
3. Results
3.1. Combined argatroban/NORel polymer characteristics
It is well described that the synthesis and use of the NO donor, DBHD/N2O2, in ECLS circuits leads to improved hemocompatibility by the ultimate preservation of circulating platelets and maintenance of their normal function via the released NO (NORel) [3, 5, 17]. However, this NORel coating alone does not prevent the activation of the intrinsic coagulation pathway and the formation of fibrin clots which are present in the NORel circuits of the rabbit extracorporeal circulation (ECC) model. Immobilizing anticoagulants such as heparin or the direct thrombin inhibitor, argatroban, to polymer coatings and then combined with the NORel coating could potentially eliminate any clot formation in extracorporeal devices.
The synthetic procedure to link argatroban to the E2As polymer, was mediated through disocyanate compounds that reacted with the secondary amine of E2As and the primary amine of argatroban (Figure 4). Isocyanates elicit polyadditive reactions with secondary amines in polyurethanes (unpublished findings) which make urethanes readily dynamic by replacing the secondary amine with a larger amine that has potential to bind biologics such as polyethylene glycol (PEG) or a direct thrombin inhibitor like argatroban. In this study, two disocyanate linking agents were used to tether argatroban to the E2As backbone, namely, HMDI or PEG-DI. Both linkers were similarly reacted with E2As for 2 h at RT and the resultant intermediate product was found to be completely soluble in THF. These intermediate products which had a single isocyanate functional group remaining was then reacted with 30 μmoles argatroban for 24 h at RT and the final product was also found to be soluble in THF. In order to determine if the linker and then argatroban were actually part of the intermediate and then final products FTIR was performed after each synthetic step. The infrared spectra of HMDI or PEG-DI/E2As intermediates and argatroban-linked E2As polymers are shown in Figures 5A and 5B, respectively. For both HMDI-linked (Figure 5A) and PEG-DI-linked E2As (Figure 5B), peaks at 2250 cm−1 are due to the free NCO group of each linker while the addition of argatroban to either linked E2As polymer have broad peaks around 3300 cm−1 which indicates the primary and secondary amines in argatroban as well as hydroxyl groups in the PEG-DI-linked argatroban polymer. There appears to be less free NCO peak in the argatroban/PEG-DI/E2As spectrum than in the argatroban/HMDI/E2As spectrum.
Figure 5.
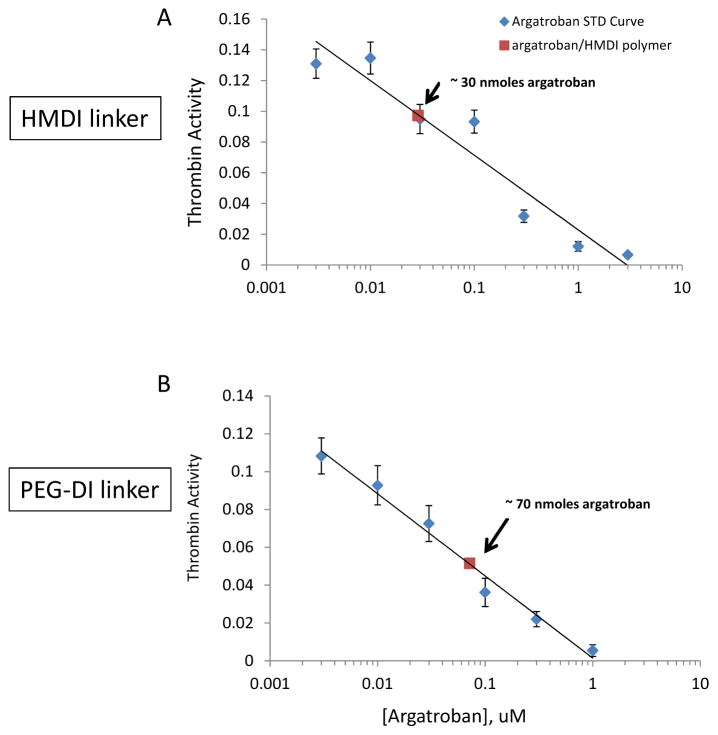
In vitro antithrombin assay using a tripeptide chromogenic substrate. Antithrombin effects of argatroban/HMDI/E2As polymer (A) or argatroban/PEG-DI/E2As polymer (B) on 0.03 U/ml human thrombin activity. Both HMDI- and PEG-DI-linked argatroban activity was compared to a standard curve of free argatroban in a 96-well format using ultraviolet absorption via microplate reader. The total moles of argatroban bound was determined from the free argatroban standard curve.
To determine if the immobilized argatroban in either HMDI- or PEG-DI-linked E2As polymers maintained its antithrombin activity, an in vitro chromogenic assay was done using a substrate that mimicked fibrinogen, the natural substrate for thrombin. A standard curve to free argatroban (0–3000 nM) was used to determine if the argatroban was first able to inhibit thrombin and then calculate the mole level for this inhibition. Figure 6A shows that immobilized argatroban to HMDI-linked E2As inhibited the activity of 0.03 U/ml human thrombin to a level that was equal to about 30 nmoles of free argatroban (Day 0). The level of thrombin inhibition for the immobilized argatroban/PEG-DI/E2As polymer was near 70 nmoles of free argatroban (Day 0).
Figure 6.
NO releasing (NORel) fabrication and top coat of argatroban-immobilized E2As polyurethane. Schematic of polymer containing a lipophilic diazeniumdiolated dibutylhexanediamine (DBHD/N2O2) and incorporated at 25 wt % into E2As polymer was coated as active layer on inner surface of PVC tubing. This was coated as an active layer on PVC tubing. The NORel coating was then top coated with the argatroban-immobilized E2As bulk polymer as a single layer (structure of argatroban/HMDI/E2As shown below arrow). The argatroban/PEG-DI/E2As bulk polymer is similar in structure as the shown HMDI-linked polymer except the C6 portion of linker is replaced by a diisocyanate linker that contains a 36 mer repeat unit of PEG.
3.2. Effects of the combined argatroban/NORel-coated ECC on rabbit hemodynamics, thrombus formation and thrombin clotting time
Without systemic heparin, previous work has shown that the control ECC with no active agents clots within four hours, is associated with a 50–70% decrease in platelet count and a 20% decrease in plasma fibrinogen [3]. Hemodynamic effects of the combined immobilized argatroban/HMDI/NORel polymer over 4 h of blood exposure in the rabbit model of thrombogenicity are shown in Table 1. Similar hemodynamic effects were observed with the combined immobilized argatroban/PEG-DI/NORel polymer over 4 h in the same rabbit model (data not shown). Hemodynamic effects remained near normal for both HMDI- or PEG-DI-linked argatroban controls and combined argatroban/NORel polymer coated ECCs. Mean arterial blood pressure (MAP) within two hours on ECC significantly fell when using the combined argatroban/HMDI/NORel group but maintained these lower levels for 4 h. This maintained MAP is due to the continuous IV fluid maintenance at 10 ml/kg/hr. Heart rate for the combined argatroban/HMDI/NORel and control ECC groups were unchanged over the 4 h. The blood flow through the ECC was dependent upon MAP and after 4 h remained unchanged from baseline due to the added IV fluids. Not unexpected, the activated clotting time (ACT) after 4 h on the combined argatroban/HMDI/NORel or control polymer ECCs increases, probably due to the increase in intravascular fluids, i.e., hemodilution effect. The total white blood cell (WBC) count fell over the 4 h for all ECC group of animals. The total WBC count is composed of lymphocytes, monocytes and granulocytes. As mentioned before, the combined argatroban/PEG-DI/NORel and its control had similar effects on hemodynamics as the combined argatroban/HMDI/NORel coated ECC.
TABLE 1.
Combination of NO releasing polymer (NORel) plus top coat of immobilized argatroban/HMDI on hemodynamic parameters in the rabbit thrombogenicity model using extracorporeal circuits (ECC).
| Treatment | Parameter | Baselinet | Time on ECC (hours)
|
|||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| Argatroban bound control polymer ECC | MAP | 74 ± 2 (42) | 40 ± 1 (6)* | 40 ± 2 (6)* | 39 ± 2 (6)* | 40 ± 1 (6)* |
| HR | 219 ± 6 (42) | 225 ± 14 (6) | 222 ± 15 (6) | 223 ± 15 (6) | 213 ± 13 (6) | |
| ECC BF | 96 ± 3 (42) | 65 ± 8 (6)* | 62 ± 4 (6)* | 61 ± 6 (6)* | 65 ± 8 (6)* | |
| ACT | 163 ± 3 (42) | 176 ± 8 (6) | 183 ± 6 (6)* | 200 ± 7 (6)* | 218 ± 10 (6)* | |
| WBC count | 6003 ± 271 (42) | 2935 ± 331 (6) | 2499 ± 237 (6)* | 2655 ± 222 (6)* | 2660 ± 437 (6)* | |
|
| ||||||
| NORel polymer ECC + bound argatroban | MAP | 74 ± 2 (42) | 37 ± 1 (6)* | 40 ± 1 (6)* | 41 ± 11 (6)* | 40 ± 1 (6)* |
| HR | 219 ± 6 (42) | 211 ± 10 (6) | 208 ± 8 (6) | 207 ± 7 (6) | 212 ± 6 (6) | |
| ECC BF | 96 ± 3 (42) | 90 ± 3 (6) | 93 ± 3 (6) | 97 ± 5 (6) | 99 ± 5 (6) | |
| ACT | 163 ± 3 (42) | 174 ± 5 (6) | 185 ± 2 (6)* | 194 ± 4 (6)* | 213 ± 5 (6)* | |
| WBC count | 6003 ± 271 (42) | 2541 ± 99 (6)* | 2979 ± 389 (6)* | 2890 ± 606 (6)* | 3383 ± 783 (6)* | |
Values are means ± SEM. ()= n size.
Values are prior to ECC placement. MAP= mean arterial pressure (mmHg), HR= heart rate (beats/min), BF= blood flow (ml/min), ACT= activated clotting time (sec), WBC= white blood cells.
p<0.05 vs baseline; Wilcoxon/Kruskal Wallis rank nonparametric test with multiple rank sum comparisons.
To ascertain the differential formation of thrombus in the thrombogenicity chamber (i.e., the 3/8 inch ID Tygon tubing 8 cm in length) of the combined argatroban/HMDI or PEG-DI/NORel polymer-coated and their respective control ECCs, 2-dimensional (2D) image analysis was performed after 4 h of blood exposure. The thrombus area was analyzed by using the Image J imaging software and represents the 2D area of thrombus formation (cm2) in each tubing chamber [3]. These thrombi area measurements were quantitated as shown in Figure 7. The thrombus area of the combined argatroban/HMDI/NORel was significantly smaller when compared to the combined argatroban/PEG-DI/NORel and the control polymer ECCs. Interestingly, the combined argatroban/HMDI/NORel ECC had a significant reduction in the thrombus formation over the NORel control that virtually no thrombi were observed in the thrombogenicity chamber, i.e., 90% thrombi reduction over 4 h control. However, the combined argatroban/PEG-DI/NORel coated ECC, which had a significant 86% reduction in thrombi versus the 4 h control ECC, formed as much thrombus as the NORel control (16% thrombi reduction over NORel control). Thus, only when HMDI is used to link argatroban to polymer and combined with NORel in ECC can a significant reduction in thrombus formation be observed over the combined argatroban/PEG-DI/NORel coated ECC. This suggests that the length of the linker on ECC surface could mediate the ultimate level of thrombus formation.
Figure 7.
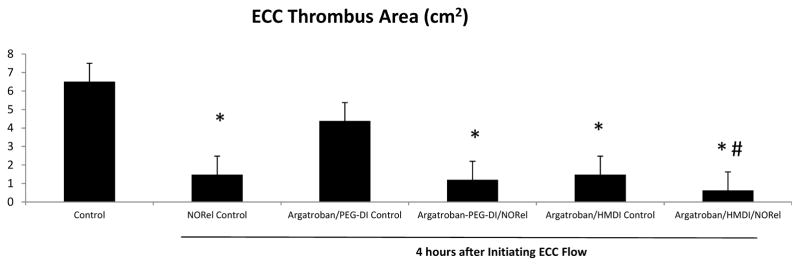
Thrombus formation of combined argatroban/HMDI or PEG-DI/NORel, argatroban and NORl alone and no argatroban and no NORel control ECCs after 4 hours blood exposure in the rabbit thrombogenicity model. Quantitation of the thrombus area (cm2) as calculated using ImageJ software from NIH. The data are means ± SEM. * = p<0.05, all versus baseline; #= p<0.05, combined argatroban/HMDI/NORel polymer coated ECC versus all other ECCs after 4 h.
From a safety aspect, determining the leaching of the immobilized argatroban from the polymer surface is quite important. The coagulation assay for direct thrombin clotting time (s) was run on the rabbit plasma after the 4 h blood exposure of all ECCs. As shown in Figure 8, none of the argatroban-containing ECCs had any increases in the thrombin clotting time. All the times were actually reduced compared to baseline. This indicates that argatroban was covalently bound to the E2AS/linker complex with minimum or no leaching.
Figure 8.
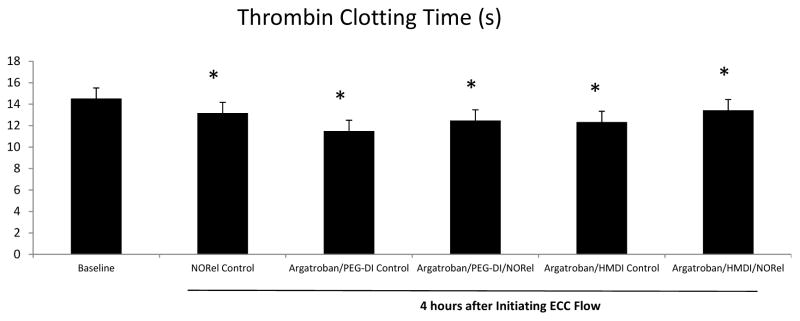
Effects of combined argatroban/HMDI or PEG-DI/NORel polymers on thrombin clotting time (TCT) after 4 h blood exposure in rabbit thrombogenicity model. TCT was measured on a Siemens BCS coagulation analyzer and expressed in seconds (s). The data are means ± SEM. *p < 0.05, baseline vs all polymer pertubations.
3.3. Effects of combined argatroban/HMDI or PEG-DI/NORel on rabbit platelet function and surface receptor expressions
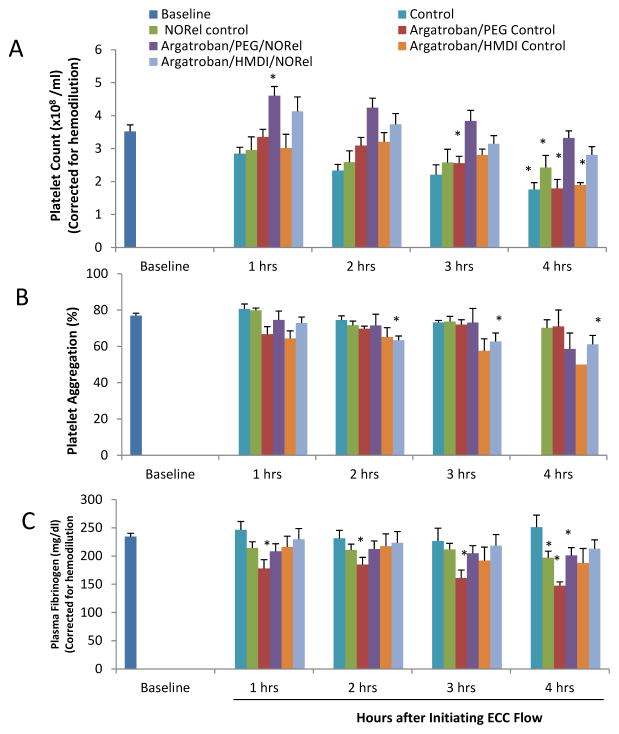
Platelet function during exposure to the combined argatroban/HMDI or PEG-DI/NORel and control polymer-coated ECC was assessed by observing platelet count (Figure 9A), the level of platelet aggregation, which determines the level platelets can respond to stimuli such as collagen or ADP (Figure 9B) and the level of plasma fibrinogen, which binds platelets during aggregation (Figure 9C). Platelet count, platelet aggregation and plasma fibrinogen levels were corrected for hemodilution due to the added IV fluids. The combined argatroban/HMDI or PEG-DI/NORel showed the greatest preservation of platelet count over the course of the 4 h blood exposure while the non-combined ECCs showed a time-dependent loss in platelet count. The combined argatroban/HMDI or PEG-DI/NORel polymer ECCs were able to maintain platelet counts to near 90% or greater even at 4 h of blood exposure when compared to the non-combined ECCs (Figure 9A). In Figure 9C, plasma fibrinogen levels are shown to be essentially unchanged in the combined argatroban/HMDI/NORel ECCs compared to non-combined ECCs. Interestingly, there was significant reduction between the combined argatroban/PEG-DI control coated ECCs and the 4 h combined argatroban/PEG-DI/NORel polymer ECC in their ability to preserve plasma fibrinogen at any time point.
Figure 9.
Time-dependent effects of NOGen polymer ECC on rabbit platelet count (A), platelet aggregometry as measured by optical turbidity (B) and plasma fibrinogen levels (C) after 4 h of blood exposure in rabbit thrombogenicity model. Platelet rich plasma was prepared and the aggregation initiated by 10 μg/ml collagen. Data is the mean ± SEM. * = p<0.05, baseline vs all coated ECCs.
The percent of platelet functional aggregation, as determined by ex vivo collagen (40 μg/ml) stimulation of PRP, was measured by optical turbidity (Figure 8B). The ability of platelets to aggregate in response to exogenous collagen was significantly inhibited with the argatroban/HMDI control ECCs where only 1 out of 6 circuits was able to measure aggregation. However, the combining of NORel polymer with the argatroban/HMDI polymer ECC maintained their ability to aggregate upon collagen stimulation, especially at the 4 h time points, demonstrating that the effects are localized.
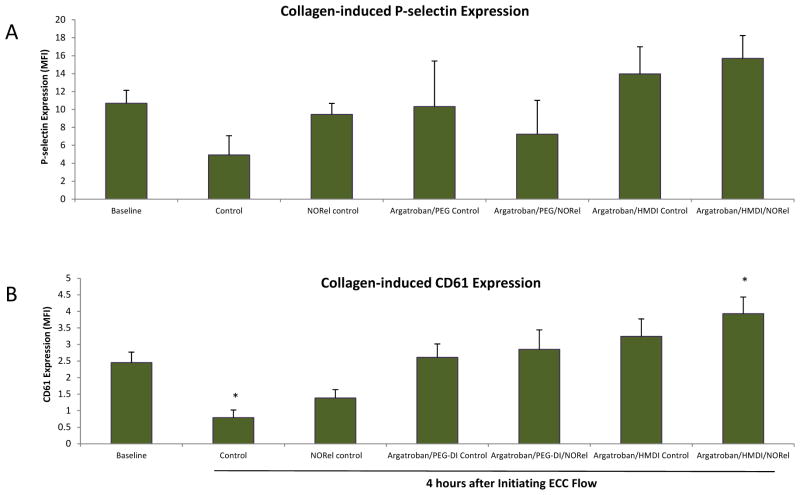
To ascertain if the observed reduction in platelet aggregation with the combined argatroban/HMDI or PEG-DI/NORel polymer ECCs after 4 h blood exposure was due to direct effects on platelet activation, expression of the platelet membrane adhesion glycoprotein, P-selectin (CD62P), was measured by fluorescence-activated cell sorting (FACS) analysis. When platelets become activated, the surface expression of P-selectin increases [18]. As shown in Figure 10A, 40 μg/ml collagen significantly stimulated an increase in P-selectin expression at baseline and after 4 h for all argatroban control and combined argatroban/NORel coated ECC groups. The collagen-stimulated P-selectin expression for the 4 h time point for the no argatroban or NORel in E2As (i.e., raw control) had a marked attenuation in P-selectin of 4.9 ± 2.2 mean fluorescence intensity (MFI) from the collagen-stimulated baseline (no ECC) levels of 10.7 ± 1.5 MFI.
Figure 10.
Fluorescent-activated cell sorting (FACS) analysis for circulating platelet P-selectin (CD62P) after 4 h blood exposure with combined argatroban/HMDI or PEG-DI/NORel or all control polymer-coated ECCs. (A) Platelet P-selectin mean fluorescence intensity (MFI) after 4 h on ECC with or without 40 μg/ml collagen stimulation in NOGen and control polymers. (B) Platelet IIIa (CD61), subunit of the platelet fibrinogen receptor, mean fluorescence intensity (MFI) after 4 h on ECC with or without 40 μg/ml collagen stimulation in combined argatroban/HMDI or PEG-DI/NORel or all control polymer-coated ECCs. The data are means ± SEM. * = p<0.05, collagen-stimulated for baseline vs collagen-stimulated for control ECC and collagen-stimulated for combined argatroban/HMDI or PEG-DI/NORel or all control polymer-coated ECCs. The specific MFI data is after the isotype control value was subtracted from each P-selectin MFI value. All FACS analyses used the gated FSC/SSC plot for platelets and 100 μl of diluted whole rabbit blood (1:100) was used for each determination.
In order to understand if the combined argatroban/HMDI or PEG-DI/NORel polymer can induce changes in the IIb/IIIa (fibrinogen) receptor, a specific antibody to IIIa (CD61) was used to evaluate platelet surface expression of IIb/IIIa via flow cytometry. As shown in Figure 10B, the platelet IIIa surface expression after 4 h for all combined argatroban/PEG-DI/NORel polymer, argatroban or NORel groups followed a similar pattern as the P-selectin expression in that they were unchanged compared to the no ECC baseline levels with 40 μg/ml exogenous collagen. The IIIa expression, however, significantly increased with 40 μg/ml exogenous collagen at combined argatroban/HMDI/NORel ECC group while the no additive (raw) control ECC group was significantly decreased in collagen-stimulated IIIa expression (Figure 10B).
3.4. Effects of combined argatroban/HMDI or PEG-DI/NORel polymer on rabbit surface receptor expressions of monocytic integrin, CD11b, and the constitutive monocyte-specific receptor, CD14
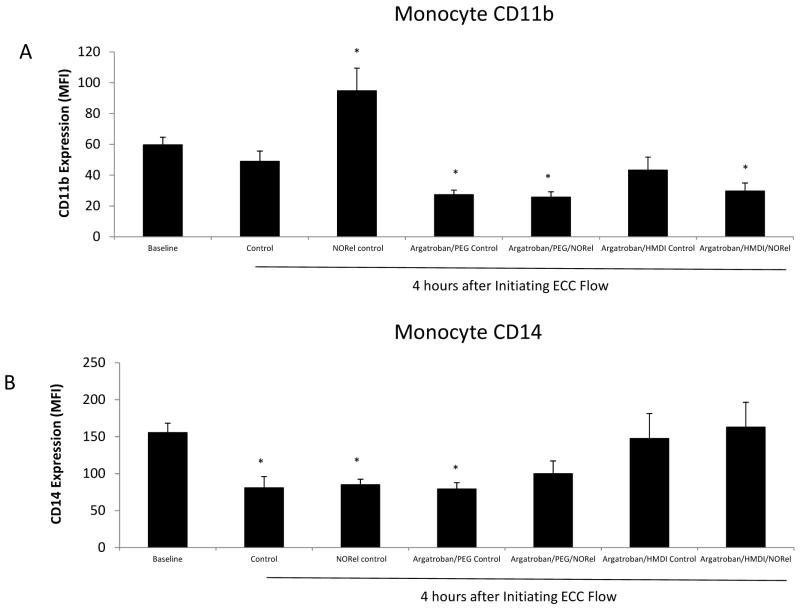
To determine if the combined argatroban/HMDI or PEG-DI/NORel polymer in the ECC can increase the surface integrin, CD11b, expression on circulating monocytes that translates into activated monocytes, FACS analysis of rabbit whole blood at baseline (no ECC) and after 4 h of circuit blood exposure was performed. An increase in CD11b expression on circulating monocytes indicates a response to an early inflammatory event [3, 19, 20]. The CD11b expression on rabbit monocytes was observed to significantly increase 1.6-fold after 4 h blood exposure to the NORel alone polymer ECC compared to no ECC baseline, but all combined argatroban/HMDI or PEG-DI/NORel or non-combined polymer ECCs exhibited no change or significant decreases in the CD11b expression from the no ECC baseline situation (Figure 11A). If the circulating monocytes are becoming activated, as indicated by the increase in CD11b expression, then the number of circulating monocytes as marked by the constitutive surface receptor, CD14, should be decreased. After 4 h of blood exposure the raw control ECC, NORel control and argatroban/PEG-DI control all showed a significant decrease in CD14 expression indicating a loss of circulating monocytes compared to the no ECC baseline levels (Figure 11B). The combined argatroban/HMDI or PEG-DI/NORel ECCs showed no change from baseline in the number of circulating monocytes after 4 h of blood exposure.
Figure 11.
Fluorescent-activated cell sorting (FACS) analysis for circulating monocytes after 4 h blood exposure with combined argatroban/HMDI or PEG-DI/NORel or all control polymer-coated ECCs. (A), Monocyte CD11b mean fluorescence intensity (MFI) after 4 h on ECC in NOGen and control polymers. (B) Monocyte CD14 (monocyte specific marker) mean fluorescence intensity (MFI) after 4 h on ECC in combined argatroban/HMDI or PEG-DI/NORel or all control polymers. The data are means ± SEM. * = p<0.05, baseline vs combined argatroban/HMDI or PEG-DI/NORel or all control polymer-coated ECCs. The specific MFI data is after the isotype control value was subtracted from each CD11b or CD14 MFI value. All FACS analyses used the gated FSC/SSC plot for monocytes and 100 μl of whole rabbit blood was used for each determination.
4. Discussion
In vivo testing of the combined argatroban and NORel-containing polymer coatings in an extracorporeal circuit demonstrated that these combined polymers can generate NO, preserve platelet count, significantly attenuate ECC thrombus formation, maintain normal platelet function (i.e., aggregation and P-selectin expression) in response to exogenous collagen and maintain monocyte inactivation after at least 4 h of blood exposure in a rabbit model of thrombogenicity. These combined argatroban/NORel polymer coatings for ECLS circuits have the strong advantage over the current heparin-coated ECCs in that they can be used in patients who have the rare, but devastating allergy to heparin or HITS. Additionally, even though both HMDI- or PEG-DI-linked argatroban polymer combined with the NORel coating had minimal ECC thrombus formation, the HMDI-linked argatroban polymer combined with NORel significantly prevented ECC thrombus formation compared to all other coatings after the 4 h of blood exposure. The NORel control in this study had shown a 75% reduction in ECC thrombus formation but combining NORel with the immobilized direct thrombin inhibitor, argatroban, further reduced clots by an additional 15% (90% reduction in total) in the extracorporeal circuit.
Previous work from our laboratory and other investigators have established that NO releasing polymers (NORel) can prevent loss of circulating platelets and monocytes, prevent up to 75% of ECC thrombus formation and maintain preservation of platelet function to normal exogenous stimuli by inhibiting ECC-induced activation [1,5,26–32]. Together, these pieces of evidence provide a strong importance for NO in defining, in part, the hemocompatibility of biomaterials used in ECLS and confirms why normal vascular endothelium provides such a beneficial nonthrombogenic surface. However, even though the NORel polymer provides great nonthrombogenic properties in ECCs through its antiplatelet effects, NORel alone still cannot inhibit the fibrin formation due to thrombin (i.e., the coagulation contribution to hemostasis (Figure 2)) during blood exposure in ECLS circuits.
Argatroban is a synthetic, small molecule, inhibitor of thrombin that has become more important in playing a significant role in systemic anticoagulation due to limitations of the most frequently used anticoagulant, heparin, in ECLS circuits [11]. Heparin’s limitations such as chemical heterogeneity and widespread binding to proteins and endothelial cells, in addition to several adverse events as the heparin-induced thrombocytopenia (HIT), prompted an alternative design of a low molecular weight, selective direct inhibitor of thrombin. Argatroban is a small molecule (MW 509) that possesses unique properties that are superior to heparin because of selectivity for the catalytic site of thrombin, ability to bind and inhibit clot-bound thrombin, short half-life and reversible nature of binding as well as a predictable dose–response relationship [21]. In the past two decades heparin has been immobilized onto plastic surfaces of catheters and small lengths of medical tubing with hope to reduce many of the systemic anticoagulation limitations [22–25]. However, many clinical procedures utilizing heparin-coated tubing have also found equivocal results quite possibly due to the indirect thrombin inhibition through patient-variable antithrombin III (ATIII) levels [26]. Immobilizing an anticoagulant that directly inhibits thrombin, both circulating and clot-bound, would provide ECLS therapy with a superior alternative.
Chemically, argatroban is the dipeptide between arginine and 4-methyl-2-piperidine carboxylic acid; one of the NH2 group of arginine is bonded to a methyltetrahydroquinoline sulfonyl group. The second NH2 group of arginine was the site used in this paper to immobilize argatroban to the plastic surface of the arterio-venous (AV) shunt (Figure 1, circled in red). Using linking compounds that have diisocyanate groups, the free primary amine of argatroban and the secondary amine in the polyurethane E2As are covalently bonded through nucleophilic addition reactions forming amide bonds [27]. These reactions use the highly reactive isocyanate groups of the bifunctional reagents, HMDI and the PEG (36 mer)-containing disocyanate, that were used in this paper to link argatroban onto the E2As polymer. One disadvantage with bifunctional reagents is any prolonged reaction can yield an insoluble product in the E2As solvent, THF, indicating cross-linking. A modified reaction procedure of Balakrishnan et al. was used in this work [28]. As shown in Figure 3 the reaction of either diisocyanate linker was done for 2 h and the resultant product was still soluble in THF after precipitation and washing with hexanes. FTIR spectra of the product from the first reaction confirmed that an isocyanate group was grafted onto the E2As polymer by the peak at 2250 cm2 which was not present in the base E2As polymer (Figure 4). The second reaction which linked argatroban via its free primary amine to the remaining isocyanate of the grafted HMDI or PEG-DI to the E2As polymer was carried out for 24 h at RT. It was noticed that a slight opaque property occurred with the reaction after 24 h. Concern developed that this resultant product would not be soluble in THF after precipitation and copious washing with deionized water. However, the argatroban-linked E2As product was soluble in the THF solvent and FTIR analysis showed that a broad peak around 3300 cm2 which was seen in IR spectrum with argatroban alone (data not shown) indicated that this product contained argatroban linked to E2As. The amount of argatroban used in the second reaction was 30 μmoles which was calculated to bind to either grafted linker in excess. Of importance with this bulk solution modification of E2As or similar polymers with linking argatroban or any anticoagulant instead of post-fabrication modification is the cost effectiveness and easier manufacture of blood/tissue compatible medical devices especially the more disposable types.
To best determine if argatroban bound to the E2As polymer and to what antithrombin activity level was present in the polymer, the in vitro antithrombin chromogenic assay was done. The level of antithrombin activity was estimated through a standard curve of free argatroban with a concentration range of 0–3000 nmoles. Argatroban bound to the two modified E2As polymers at an amount of 30 nmoles with the HMDI-linked E2As and 70 nmoles bound to the PEG-DI-linked E2As. These levels of antithrombin activity of the two argatroban-immobilized polymers proved that argatroban had bound to the polymers but some might have been in an unbound free state within polymers. Future studies will be done to determine if all the reacted argatroban was bound or some remained unbound. However, even if some of the argatroban was unbound in the polymers and had leached out over the 4 h blood exposure in the rabbit thrombogenicity model, the thrombin clotting time was normal indicating no thrombin inhibition even if some argatroban had leached out of the polymers. Previous work has shown that immobilizing argatroban at the free primary amine to polymers or as a dimer of argatroban does not affect or affects minimally the antithrombin property of argatroban [29–31]. In determining if the free primary amine of arginine in argatroban was necessary for the antithrombin activity, we found that linking two molecules of argatroban using the diisocynate, HMDI, did not significantly reduce the inhibitory effect of argatroban on human thrombin activity (unpublished findings). This result left the 4-methyl-2-piperidine carboxylic acid and methyltetrahydroquinoline sulfonyl groups available for specific and reversible binding into the active site of thrombin as shown by X-ray crystallography. Two pockets in the active site of thrombin, the ‘P’ and ‘D’ pockets, have been shown to bind the 4-methyl-2-piperidine carboxylic acid group and the methyltetrahydroquinoline sulfonyl group, respectively [32]. These results confirmed that the antithrombin activity observed in this argatroban-immobilized polymer was due to the binding of these two structural groups binding to the active site of thrombin.
With the argatroban-immobilized E2As polymer confirmed to inhibit thrombin activity, this polymer coating was combined with the NORel polymer coating in the A-V shunt of the 4 h rabbit thrombogenicity model. The NORel/E2As polymer was shown to have a maximum NO release level of approx. 6 × 10−10 mol cm−2 min−1 which was comparable to NO flux reported previously from our laboratory [33]. Interestingly, the NO flux was not adversely affected by the top coating of the argatroban-immobilized polymer (6 × 10−10 before top coat and 6.5 × 10−10 mol cm−2 min−1 after top coat). Additionally, 4 h after blood exposure, the NO release flux still remained near pre-blood exposure levels (5.7 × 10−10 mol cm−2 min−1). With the combined antithrombin and antiplatelet polymer coatings confirmed to be functioning, the effects of this combined coating on thrombus formation, platelet count and function and early inflammatory responses through monocyte CD11b upregulation was determined.
The effects of the combined argatroban/NORel coated ECC on hemodynamic and ECC flow parameters in the rabbit thrombogenicity model remained unremarkable compared to baseline readings prior to ECC exposure. Mean arterial pressure fall slightly after ECC exposure up to 4 h but this was counteracted with giving IV maintenance fluids at a rate of 10 ml/kg/h. The argatroban polymer alone had similar unremarkable effects compared to baseline as the combined argatroban/NORel polymer.
The effects on thrombus formation in the 4 h rabbit thrombogenicity model by the combined argatroban/NORel polymer coating as well as the control polymers were determined without systemic anticoagulation. For the first time the results for the combined argatroban/NORel polymer, specifically, the argatroban/HMDI/NORel polymer, had a significant reduction in the thrombus area that was present in the thrombogenicity ECC chamber after 4 h of blood exposure compared to any of the other polymer coatings even the combined argatroban/PEG-DI/NORel coating. The results confirm our previous data that NORel alone significantly attenuates the clot area in the ECC [3, 33] but the added antithrombin inhibition by argatroban essentially prevents any further clotting by reducing fibrin formation which none of the NO releasing polymers had any effect on thrombin activity as indicated by no change in plasma thrombin clotting time (Figure 8). In addition, the argatroban/PEG-DI/NORel combined polymer showed no better thrombus area reduction than NORel polymer alone. Previous work using PEG as part of the coated surface of ECCs has demonstrated that there is a reduced amount of platelet adhesion due to reduce protein adsorption [28, 34–38]. It was hypothesized that including PEG in the linked argatroban would possibly also reduce the adsorbed plasma proteins to the ECC surface as well as be antithrombin and antiplatelet. The length of the PEG moiety could influence the effectiveness of the immobilized argatroban to inhibit thrombin and reduce clot formation. These mechanisms will be part of future studies.
To understand the role of the platelet and monocyte in the combined argatroban/HMDI and PEG-DI/NORel polymer effects in reducing the thrombus formation, platelet count, aggregation, P-selectin expression and monocyte CD11b proinflammatory expression were determined. Platelet count and plasma fibrinogen levels remained unchanged from baseline for both the combined argatroban/HMDI or PEG-DI/NORel polymers and the level of functionality as measured via aggregometry of the platelets remained above 60%. These results confirm our previous data using NORel polymer-coated ECCs [3, 4, 23, 39–42] and indicate that, with minimal fibrin clot formation, platelet consumption was significantly minimized due to the presence of argatroban in the two combined argatroban/HMDI or PEG-DI/NORel polymer coated ECCs and less clot for platelets to get bound into its matrix. To determine the mechanism that may drive the preservation of circulating platelets and monocytes after 4 h blood exposure in the combined argatroban/NORel ECCs, platelet membrane expression of the glycoprotein P-selectin, or CD62P, and monocyte membrane expression of the integrin, CD11b, were measured via flow cytometry.
Platelet P-selectin expression, representing an early biomarker for platelet activation, was measured when ex vivo stimulated by 40 μg/ml collagen. When the collagen-induced P-selectin expression was reduced compared to baseline then platelets were considered already activated while collagen-induced P-selectin expression being similar to baseline after 4 h blood exposure would indicate a preservation of normal function of circulating platelets. The P-selectin expression in this study showed that with no argatroban or NORel coatings the collagen-induced P-selectin expression was near a significant reduction compared to baseline but in the presence of argatroban and NORel coatings the circulating platelet function as measured via collagen-induced P-selectin was preserved indicating that locally in the ECC argatroban and NORel were able to maintain in a resting state and able to respond to an external stimulus such as collagen. Interestingly, the constitutive membrane marker for platelets, CD61, which is the β subunit of the fibrinogen receptor (i.e., IIIa), also showed reduced collagen-induced expression without argatroban and NORel present and with argatroban/NORel polymers the CD61 expression was unchanged or increased (argatroban/HMDI/NORel) compared to baseline. These results indicate that the platelet number was preserved with argatroban and NORel. Previous work also has shown that the direct thrombin inhibitor, argatroban, has attenuated P-selectin expression in platelets and preserved their number when exposed to activating stimuli or extracorporeal circulations [43–48]. It is hypothesized that circulating platelets are locally ‘anesthetized’ by the polymer released NO and any platelet-released thrombin would be inhibited by the immobilized argatroban. The slightly reduced collagen-induced P-selectin and CD61 expressions observed with the combined argatroban/PEG-DI/NORel polymer compared to the combined argatroban/HMDI/NORel might be that the PEG chain (36 mer) places the argatroban further from ECC surface and locally released thrombin is not inhibited as much as when argatroban is linked to polymer with HMDI. This is under further investigation.
Finally, the combined argatroban/HMDI or PEG-DI/NORel polymer coated ECCs reduced the monocyte CD11b expression after 4 h blood exposure in the rabbit thrombogenicity model compared to no ECC baseline. Argatroban alone polymer coated ECC also had reduced CD11b expression while the NORel alone polymer appeared to increase CD11b compared to baseline. These results indicate that argatroban play a role in inhibiting CD11b expression on monocytes. The integrin CD11b is an early biomarker for inflammatory initiation and is upregulated, in part, on leukocytes by thrombin [49, 50]. Therefore, argatroban, which inhibits thrombin activity, can indirectly reduce the level of monocyte CD11b expression and the initiation of inflammation. Previous work has shown that argatroban does reduce CD11b expression on monocytes and other leukocytes [51] of which our results in this paper confirm. Additionally, it was found that the constitutive lipopolysaccharide receptor, CD14 expression was also normalized in the combined argatroban/HMDI or PEG-DI/NORel polymer coated ECCs near baseline levels. CD14 expression is only expressed on monocytes and the expression wanes when monocytes differentiate into macrophages upon activation. The level of CD14 expression therefore confirms that WBC counts are essentially unchanged from baseline in the combined polymer ECCs. These data indicate that argatroban plays a significant role in eliminating any thrombin released from circulating platelets that NO alone cannot attenuate.
5. Conclusions
This study has demonstrated that coating a solution of E2As polyurethane containing immobilized argatroban and NORel on PVC tubing can release NO and prevent up to 90% of thrombus formation for at least 4 h in a rabbit model of extracorporeal circulation. The innovative part of this combined coating is that the immobilization of argatroban was modified in bulk solution and not immobilized post-ECC fabrication. These results suggest that the combined argatroban/HMDI/NORel polymer coating preserves platelets in blood exposure to ECCs to a better degree than the argatroban/PEGDI/NORel coating, NORel alone or argatroban alone. These combined antithrombin, NO-mediated antiplatelet effects were shown to improve thromboresistance of the combined argatroban and NORel polymer-coated ECCs and have brought ECLS circuit coatings closer to mimicking intact vascular endothelium in preventing thrombogenicity.
Acknowledgments
The authors declare this work is supported by National Institutes of Health grants R21EB016236 and K25HL111213 and the Federal Drug Administration, Grant #2P50FD003787-03. The authors wish to thank AorTech International for the gift of the Elast-Eon E2As polymer.
Footnotes
The authors confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ranucci M, Pazzaglia A, Isgro G, Cazzaniga A, Ditta A, Boncilli A, et al. Closed, phosphorylcholine-coated circuit and reduction of systemic heparinization for cardiopulmonary bypass: the intraoperative ECMO concept. Int J Artif Organs. 2002;25:875–81. doi: 10.1177/039139880202500910. [DOI] [PubMed] [Google Scholar]
- 2.Conn G, Kidane AG, Punshon G, Kannan RY, Hamilton G, Seifalian AM. Is there an alternative to systemic anticoagulation, as related to interventional biomedical devices? Expert Rev Med Devices. 2006;3:245–61. doi: 10.1586/17434440.3.2.245. [DOI] [PubMed] [Google Scholar]
- 3.Major TC, Brant DO, Reynolds MM, Bartlett RH, Meyerhoff ME, Handa H, et al. The attenuation of platelet and monocyte activation in a rabbit model of extracorporeal circulation by a nitric oxide releasing polymer. Biomaterials. 2010;31:2736–45. doi: 10.1016/j.biomaterials.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skrzypchak AM, Lafayette NG, Bartlett RH, Zhou Z, Frost MC, Meyerhoff ME, et al. Effect of varying nitric oxide release to prevent platelet consumption and preserve platelet function in an in vivo model of extracorporeal circulation. Perfusion. 2007;22:193–200. doi: 10.1177/0267659107080877. [DOI] [PubMed] [Google Scholar]
- 5.Major TC, Handa H, Brisbois EJ, Reynolds MM, Annich GM, Meyerhoff ME, et al. The mediation of platelet quiescence by NO-releasing polymers via cGMP-induced serine 239 phosphorylation of vasodilator-stimulated phosphoprotein. Biomaterials. 2013;34:8086–96. doi: 10.1016/j.biomaterials.2013.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang H, Annich GM, Miskulin J, Osterholzer K, Merz SI, Bartlett RH, et al. Nitric oxide releasing silicone rubbers with improved blood compatibility: preparation, characterization, and in vivo evaluation. Biomaterials. 2002;23:1485–94. doi: 10.1016/s0142-9612(01)00274-5. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds MM, Frost MC, Meyerhoff ME. Nitric oxide-releasing hydrophobic polymers: preparation, characterization, and potential biomedical applications. Free Radic Biol Med. 2004;37:926–36. doi: 10.1016/j.freeradbiomed.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 8.Frost MC, Reynolds MM, Meyerhoff ME. Polymers incorporating nitric oxide releasing/generating substances for improved biocompatibility of blood-contacting medical devices. Biomaterials. 2005;26:1685–93. doi: 10.1016/j.biomaterials.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Annich GM, Meinhardt JP, Mowery KA, Ashton BA, Merz SI, Hirschl RB, et al. Reduced platelet activation and thrombosis in extracorporeal circuits coated with nitric oxide release polymers. Crit Care Med. 2000;28:915–20. doi: 10.1097/00003246-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Frank RD, Muller U, Lanzmich R, Groeger C, Floege J. Anticoagulant-free genius haemodialysis using low molecular weight heparin-coated circuits. Nephrol Dial Transplant. 2006;21:1013–8. doi: 10.1093/ndt/gfi293. [DOI] [PubMed] [Google Scholar]
- 11.Bush LR. Argatroban, A selective, potent thrombin inhibitor. Cardiovascular Drug Reviews. 1991;9:247–63. [Google Scholar]
- 12.Sabatine MS, Tu TM, Jang IK. Combination of a direct thrombin inhibitor and a platelet glycoprotein IIb/IIIa blocking peptide facilitates and maintains reperfusion of platelet-rich thrombus with alteplase. J Thromb Thrombolysis. 2000;10:189–96. doi: 10.1023/a:1018722828543. [DOI] [PubMed] [Google Scholar]
- 13.Murphy KD, Galla DH, Vaughn CJ, McCrohan G, Garrisi WJ. Heparin-induced thrombocytopenia and thrombosis syndrome. Radiographics. 1998;18:111–20. doi: 10.1148/radiographics.18.1.9460112. [DOI] [PubMed] [Google Scholar]
- 14.Batchelor MM, Reoma SL, Fleser PS, Nuthakki VK, Callahan RE, Shanley CJ, et al. More lipophilic dialkyldiamine-based diazeniumdiolates: synthesis, characterization, and application in preparing thromboresistant nitric oxide release polymeric coatings. J Med Chem. 2003;46:5153–61. doi: 10.1021/jm030286t. [DOI] [PubMed] [Google Scholar]
- 15.Massaguer A, Engel P, Perez-del-Pulgar S, Bosch J, Pizcueta P. Production and characterization of monoclonal antibodies against conserved epitopes of P-selectin (CD62P) Tissue Antigens. 2000;56:117–28. doi: 10.1034/j.1399-0039.2000.560202.x. [DOI] [PubMed] [Google Scholar]
- 16.Gosselin RC, King JH, Janatpur KA, Dager WH, Larkin EC, Owings JT. Effects of pentasaccharide (fondaparinux) and direct thrombin inhibitors on coagulation testing. Arch Pathol Lab Med. 2004;128:1142–5. doi: 10.5858/2004-128-1142-EOPFAD. [DOI] [PubMed] [Google Scholar]
- 17.Handa H, Brisbois EJ, Major TC, Refahiyat L, Amoako KA, Annich GM, et al. and study of sustained nitric oxide release coating using diazeniumdiolate-doped poly(vinyl chloride) matrix with poly(lactide--glycolide) additive. J Mater Chem B Mater Biol Med. 2013;1:3578–87. doi: 10.1039/C3TB20277A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrews RK, Berndt MC. Platelet physiology and thrombosis. Thromb Res. 2004;114:447–53. doi: 10.1016/j.thromres.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 19.El-Sabbagh AM, Toomasian CJ, Toomasian JM, Ulysse G, Major T, Bartlett RH. Effect of air exposure and suction on blood cell activation and hemolysis in an in vitro cardiotomy suction model. ASAIO J. 2013;59:474–9. doi: 10.1097/MAT.0b013e31829f0e6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ilmakunnas M, Pesonen EJ, Ahonen J, Ramo J, Siitonen S, Repo H. Activation of neutrophils and monocytes by a leukocyte-depleting filter used throughout cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2005;129:851–9. doi: 10.1016/j.jtcvs.2004.07.061. [DOI] [PubMed] [Google Scholar]
- 21.Yeh RW, Jang IK. Argatroban: update. Am Heart J. 2006;151:1131–8. doi: 10.1016/j.ahj.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Clark TW, Jacobs D, Charles HW, Kovacs S, Aquino T, Erinjeri J, et al. Comparison of heparin-coated and conventional split-tip hemodialysis catheters. Cardiovasc Intervent Radiol. 2009;32:703–6. doi: 10.1007/s00270-009-9608-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu B, Gerlitz B, Grinnell BW, Meyerhoff ME. Polymeric coatings that mimic the endothelium: combining nitric oxide release with surface-bound active thrombomodulin and heparin. Biomaterials. 2007;28:4047–55. doi: 10.1016/j.biomaterials.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du YJ, Brash JL, McClung G, Berry LR, Klement P, Chan AK. Protein adsorption on polyurethane catheters modified with a novel antithrombin-heparin covalent complex. J Biomed Mater Res A. 2007;80:216–25. doi: 10.1002/jbm.a.30977. [DOI] [PubMed] [Google Scholar]
- 25.Zimmermann AK, Weber N, Aebert H, Ziemer G, Wendel HP. Effect of biopassive and bioactive surface-coatings on the hemocompatibility of membrane oxygenators. J Biomed Mater Res B Appl Biomater. 2007;80:433–9. doi: 10.1002/jbm.b.30614. [DOI] [PubMed] [Google Scholar]
- 26.Beiderlinden M, Treschan T, Gorlinger K, Peters J. Argatroban in extracorporeal membrane oxygenation. Artif Organs. 2007;31:461–5. doi: 10.1111/j.1525-1594.2007.00388.x. [DOI] [PubMed] [Google Scholar]
- 27.Ying H, Zhang Y, Cheng J. Dynamic urea bond for the design of reversible and self-healing polymers. Nat Commun. 2014;5:3218. doi: 10.1038/ncomms4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balakrishnan B, Kumar DS, Yoshida Y, Jayakrishnan A. Chemical modification of poly(vinyl chloride) resin using poly(ethylene glycol) to improve blood compatibility. Biomaterials. 2005;26:3495–502. doi: 10.1016/j.biomaterials.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 29.Richey T, Iwata H, Oowaki H, Uchida E, Matsuda S, Ikada Y. Surface modification of polyethylene balloon catheters for local drug delivery. Biomaterials. 2000;21:1057–65. doi: 10.1016/s0142-9612(99)00281-1. [DOI] [PubMed] [Google Scholar]
- 30.Kruse KR, Crowley JJ, Tanguay JF, Santos RM, Millare DS, Phillips HR, et al. Local drug delivery of argatroban from a polymeric-metallic composite stent reduces platelet deposition in a swine coronary model. Catheter Cardiovasc Interv. 1999;46:503–7. doi: 10.1002/(SICI)1522-726X(199904)46:4<503::AID-CCD25>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 31.Noguchi H, Iwata H, Ikada Y. Synthesis of monomeric and polymeric conjugates carrying a thrombin inhibitor through an ester bond. J Biomed Mater Res. 1998;39:621–9. doi: 10.1002/(sici)1097-4636(19980315)39:4<621::aid-jbm17>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 32.Banner DW, Hadvary P. Crystallographic analysis at 3.0-A resolution of the binding to human thrombin of four active site-directed inhibitors. J Biol Chem. 1991;266:20085–93. [PubMed] [Google Scholar]
- 33.Handa H, Major TC, Brisbois EJ, Amoako KA, Meyerhoff ME, Bartlett RH. Hemocompatibility comparison of biomedical grade polymers using rabbit thrombogenicity model for preparing nonthrombogenic nitric oxide releasing surfaces. J Mater Chem B Mater Biol Med. 2014;2:1059–67. doi: 10.1039/C3TB21771J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao X, Kucerka N, Nieh MP, Katsaras J, Zhu S, Brash JL, et al. Chain conformation of a new class of PEG-based thermoresponsive polymer brushes grafted on silicon as determined by neutron reflectometry. Langmuir. 2009;25:10271–8. doi: 10.1021/la901086e. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Z, Zhang M, Chen S, Horbett TA, Ratner BD, Jiang S. Blood compatibility of surfaces with superlow protein adsorption. Biomaterials. 2008;29:4285–91. doi: 10.1016/j.biomaterials.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 36.Hoffmann J, Groll J, Heuts J, Rong H, Klee D, Ziemer G, et al. Blood cell and plasma protein repellent properties of star-PEG-modified surfaces. J Biomater Sci Polym Ed. 2006;17:985–96. doi: 10.1163/156856206778366059. [DOI] [PubMed] [Google Scholar]
- 37.Feng W, Zhu S, Ishihara K, Brash JL. Protein resistant surfaces: comparison of acrylate graft polymers bearing oligo-ethylene oxide and phosphorylcholine side chains. Biointerphases. 2006;1:50. doi: 10.1116/1.2187495. [DOI] [PubMed] [Google Scholar]
- 38.Wu Y, Simonovsky FI, Ratner BD, Horbett TA. The role of adsorbed fibrinogen in platelet adhesion to polyurethane surfaces: a comparison of surface hydrophobicity, protein adsorption, monoclonal antibody binding, and platelet adhesion. J Biomed Mater Res A. 2005;74:722–38. doi: 10.1002/jbm.a.30381. [DOI] [PubMed] [Google Scholar]
- 39.Reynolds MM, Hrabie JA, Oh BK, Politis JK, Citro ML, Keefer LK, et al. Nitric oxide releasing polyurethanes with covalently linked diazeniumdiolated secondary amines. Biomacromolecules. 2006;7:987–94. doi: 10.1021/bm060028o. [DOI] [PubMed] [Google Scholar]
- 40.Zhou Z, Meyerhoff ME. Preparation and characterization of polymeric coatings with combined nitric oxide release and immobilized active heparin. Biomaterials. 2005;26:6506–17. doi: 10.1016/j.biomaterials.2005.04.046. [DOI] [PubMed] [Google Scholar]
- 41.Fleser PS, Nuthakki VK, Malinzak LE, Callahan RE, Seymour ML, Reynolds MM, et al. Nitric oxide-releasing biopolymers inhibit thrombus formation in a sheep model of arteriovenous bridge grafts. J Vasc Surg. 2004;40:803–11. doi: 10.1016/j.jvs.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Annich GM, Meinhardt JP, Mowery KA, Ashton BA, Merz SI, Hirschl RB, et al. Reduced platelet activation and thrombosis in extracorporeal circuits coated with nitric oxide release polymers. Crit Care Med. 2000;28:915–20. doi: 10.1097/00003246-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Musashi K, Kiryu J, Miyamoto K, Miyahara S, Katsuta H, Tamura H, et al. Thrombin inhibitor reduces leukocyte-endothelial cell interactions and vascular leakage after scatter laser photocoagulation. Invest Ophthalmol Vis Sci. 2005;46:2561–6. doi: 10.1167/iovs.04-1102. [DOI] [PubMed] [Google Scholar]
- 44.Kanemitsu S, Nishikawa M, Onoda K, Shimono T, Shimpo H, Yazaki A, et al. Pharmacologic platelet anesthesia by glycoprotein IIb/IIIa complex antagonist and argatroban during in vitro extracorporeal circulation. J Thorac Cardiovasc Surg. 2003;126:428–35. doi: 10.1016/s0022-5223(02)73288-1. [DOI] [PubMed] [Google Scholar]
- 45.Miyahara S, Kiryu J, Tsujikawa A, Katsuta H, Nishijima K, Miyamoto K, et al. Argatroban attenuates leukocyte- and platelet-endothelial cell interactions after transient retinal ischemia. Stroke. 2003;34:2043–9. doi: 10.1161/01.STR.0000083052.01361.3D. [DOI] [PubMed] [Google Scholar]
- 46.Amin HM, Ahmad S, Walenga JM, Hoppensteadt DA, Leitz H, Fareed J. Soluble P-selectin in human plasma: effect of anticoagulant matrix and its levels in patients with cardiovascular disorders. Clin Appl Thromb Hemost. 2000;6:71–6. doi: 10.1177/107602960000600204. [DOI] [PubMed] [Google Scholar]
- 47.Xiao Z, Theroux P. Platelet activation with unfractionated heparin at therapeutic concentrations and comparisons with a low-molecular-weight heparin and with a direct thrombin inhibitor. Circulation. 1998;97:251–6. doi: 10.1161/01.cir.97.3.251. [DOI] [PubMed] [Google Scholar]
- 48.Kaiser B, Koza M, Walenga JM, Fareed J. Flow cytometric evaluation of the effect of various thrombin inhibitors on platelet activation in whole blood. Thromb Res. 1996;82:257–63. doi: 10.1016/0049-3848(96)00072-2. [DOI] [PubMed] [Google Scholar]
- 49.Rao NV, Argyle B, Xu X, Reynolds PR, Walenga JM, Prechel M, et al. Low anticoagulant heparin targets multiple sites of inflammation, suppresses heparin-induced thrombocytopenia, and inhibits interaction of RAGE with its ligands. Am J Physiol Cell Physiol. 2010;299:C97–110. doi: 10.1152/ajpcell.00009.2010. [DOI] [PubMed] [Google Scholar]
- 50.Alberti S, Angeloni G, Tamburrelli C, Pampuch A, Izzi B, Messano L, et al. Platelet-leukocyte mixed conjugates in patients with atrial fibrillation. Platelets. 2009;20:235–41. doi: 10.1080/09537100902954370. [DOI] [PubMed] [Google Scholar]
- 51.Li N, He S, Blomback M, Hjemdahl P. Platelet activity, coagulation, and fibrinolysis during exercise in healthy males: effects of thrombin inhibition by argatroban and enoxaparin. Arterioscler Thromb Vasc Biol. 2007;27:407–13. doi: 10.1161/01.ATV.0000253906.19648.ac. [DOI] [PubMed] [Google Scholar]