Abstract
Purpose
The effects of an anticholinergic or antidiuretic agent as add-on therapy to an alpha-blocker for lower urinary tract symptoms (LUTS) according to a voiding diary in 3 days are unknown. We prospectively investigated the efficacy of an anticholinergic or antidiuretic agent as add-on therapy for nocturia in men previously treated with an alpha-blocker for LUTS.
Subjects and methods
Patients were randomly subdivided into two groups. All patients had a 4-week washout. Group A had alpha-blocker for 4 weeks, then an alpha-blocker plus an anticholinergic agent for 4 weeks, and, finally, 4 weeks of an alpha-blocker plus an antidiuretic agent. Group B had an alpha-blocker for 4 weeks, then an alpha-blocker plus an antidiuretic agent for 4 weeks, and, finally, 4 weeks of an alpha-blocker plus an anticholinergic agent. In both groups, patients were subdivided into nocturnal polyuria, decreased nocturnal bladder capacity (NBC), or nocturia by both causes subgroups. A 3-day voiding diary, total International Prostate Symptom Score (IPSS), IPSS sub-scores, Overactive Bladder Symptom Score, uroflowmetry, and post-void residual urine volume, were assessed at baseline, and at 4, 8, and 12 weeks.
Results
A total of 405 patients completed the study. During treatment, the changes from baseline in total IPSS and IPSS sub-scores were significantly decreased at 4 weeks and were maintained for 12 weeks. In the nocturnal polyuria subgroup of Groups A and B, the number of episodes of nocturia in 3 days, nocturnal urine volume, and nocturnal index were significantly decreased using an alpha-blocker plus an antidiuretic agent. In the decreased NBC subgroup of Groups A and B, IPSS storage sub-score, Overactive Bladder Symptom Score, number of episodes of nocturia in 3 days, number of episodes of urgency in 3 days, and NBC index were all significantly decreased using an alpha-blocker plus an anticholinergic agent.
Conclusion
An anticholinergic agent or antidiuretic agent as an add-on therapy in men previously treated with an alpha-blocker improves nocturia including LUTS.
Keywords: LUTS, nocturia, nocturnal bladder capacity, benign prostatic hyperplasia
Introduction
Benign prostatic hyperplasia (BPH) often causes bladder-outlet obstruction (BOO) and commonly results in lower urinary tract symptoms (LUTS). LUTS diminish quality of life by interfering with daily activities and decreasing psychological well-being. In men, LUTS are typically treated initially with agents that target the prostate, such as an alpha-blocker. However, alpha-blockers may have limited efficacy in relieving overactive bladder (OAB) symptoms.1
Storage symptoms often independently occur and persist in many men after pharmacologic treatment for BOO.2 Therefore, treatments that target the prostate often fail to alleviate OAB symptoms and may not be the most appropriate therapy for men with storage LUTS.3 Treatment with an alpha-blocker and an anticholinergic agent improves LUTS compared with an alpha-blocker alone.4–6 LUTS are one of the major causes of nocturia in older men and are associated with a decreased bladder capacity due to detrusor overactivity or high post-voiding residual volume (PVR), which results in decreased voiding volume and increased micturition frequency.7 Because BOO is a potential risk factor for nocturia, alpha-blockers are considered a potential treatment for nocturia. However, the relief of BOO is not sufficient to correct nocturia.8 While the pathophysiology of nocturia is considered multifactorial, nocturnal polyuria (NP) and decreased nocturnal bladder capacity (NBC) are the main mechanisms.9
The efficacy and safety of desmopressin in the treatment of adults with nocturia have been demonstrated in randomized trials.10–12 However, the effects of an anticholinergic agent or an antidiuretic agent as an add-on therapy to an alpha-blocker in patients with LUTS remain unknown. In the study reported here, we analyzed the efficacy of anticholinergic agent and antidiuretic agent add-on therapy for refractory nocturia in men previously treated with an alpha-blocker for LUTS, according to voiding disorders (nocturnal polyuria, decreased nocturnal bladder capacity [NBC], or nocturia by both causes subgroups).
Patients and methods
Patient selection and data collection
We obtained approval for the study from the institutional review board at our hospital. Between July 2010 and April 2013, men ≥50 years of age diagnosed with LUTS due to BOO, with a maximum urinary flow rate (Qmax) ≤15 mL/second, nocturia (≥1 void/night), and a total International Prostate Symptom Score (IPSS) ≥14 (voiding sub-score ≥8 and storage sub-score ≥6) were treated with an alpha-blocker for at least 4 weeks.13
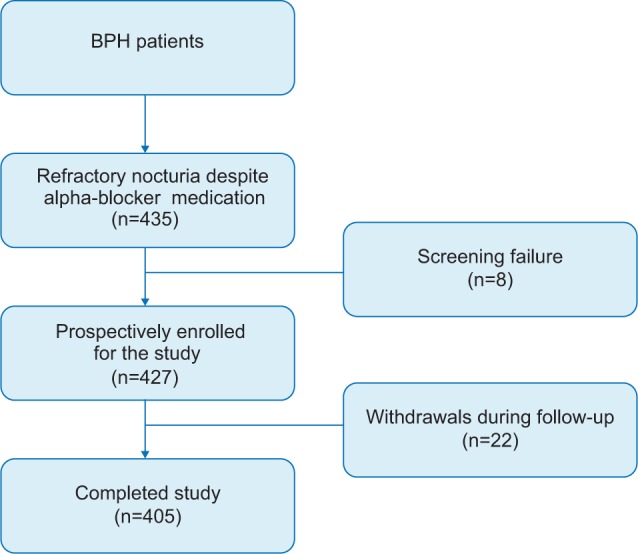
Four hundred and thirty five patients with persistent nocturia were prospectively/retrospectively enrolled after providing written informed consent. All patients had a 4-week drug washout period before enrollment. Patients were excluded if they had neurogenic bladder dysfunction, hyponatremia, uncontrolled hypertension, congestive heart failure, history of prostate surgery, interstitial cystitis, elevated prostate specific antigen, or had been previously treated with anticholinergic drugs or diuretics. Patients were randomly divided into two groups at the time of enrollment. Group A had an alpha-blocker (tamsulosin 0.2 mg) at bedtime more than 4 weeks of an alpha-blocker plus an anticholinergic agent (solifenacin 5 mg) orally at bedtime, then, finally, 4 weeks of an alpha-blocker plus an antidiuretic agent (desmopressin 0.2 mg). Group B had an alpha-blocker for 4 weeks orally at bedtime followed by 4 weeks of an alpha-blocker plus an antidiuretic agent (desmopressin 0.2 mg) orally at bedtime, then, finally, 4 weeks of an alpha-blocker plus an anticholinergic agent (solifenacin 5 mg). Patients were required to visit the outpatient clinic at the start of the study for screening and after 4, 8, and 12 weeks (Figure 1). If adverse events occurred, patients were excluded during the study without dose titration or drug resting time.
Figure 1.
Study design.
Abbreviation: BPH, benign prostate hyperplasia.
Assessment tools
All patients recorded a 3-day VD at baseline and at 4, 8, and 12 weeks. Patients in both groups were subdivided into three subgroups: NP (voided urine volume during sleep >33% of the 24-hour output), decreased NBC (NBC index is greater than 0; NBC index corresponds to the actual number of voids subtracted by the predicted number of voids; predict number of voids, nocturia index –1; nocturia index is the nocturnal urine volume divided by functional bladder capacity; and functional bladder capacity is the single largest volume voided and recorded in VD), and nocturia due to both causes. Total IPSS and IPSS sub-scores (voiding and storage) and Overactive Bladder Symptom Score (OABSS) were analyzed at baseline and at 4, 8, and 12 weeks.14 Qmax and PVR were assessed using flowmetry and ultrasound (9032A0072, Medtronic, Skovlunde and TYPE 2202; B&K Medical, Herlev, Denmark), respectively. Safety was also evaluated from reported adverse events and laboratory data.
Statistical analysis
All data are reported as the mean ± standard deviation. Data were analyzed using SPSS software (v 15.0; IBM Corporation, Armonk, NY, USA). A P-value of <0.05 was considered significant. One-way analysis of variance tests were used to compare the baseline data between the groups. Mean changes from baseline to 4 weeks, 4–8 weeks, and 4–12 weeks in total IPSS score, IPSS sub-scores, OABSS, Qmax, and PVR were analyzed using paired Student’s t-test.
Results
Among the 435 patients screened for the study, eight withdrew due to screening failure. A total of 427 patients were prospectively enrolled and 405 patients completed the study (Figure 1). Twenty-two (5.4%) patients withdrew due to adverse events. Of those, nine withdrew from worse voiding by the addition of anticholinergic agent, seven experienced dry mouth, and one experienced constipation. Four withdrew due to dizziness and hyponatremia when taking an antidiuretic agent as add-on therapy. One patient withdrew due to uncontrolled hypertension during follow-up.
The baseline characteristics of IPSS and OABSS, uroflowmetry parameters, and 3-day VD details of Groups A and B are summarized in Table 1. In Groups A and B, the NP subgroup displayed significantly higher nocturnal urine volume and OABSS, while the number of episodes of urgency in 3 days and NBC index were lower in this subgroup than in the others (P<0.05; Tables 2 and 3). In the decreased NBC subgroup, the IPSS storage sub-score was significantly higher than in the other subgroups in Groups A and B (P<0.05; Tables 2 and 3). During the treatment, the changes from baseline in total IPSS significantly decreased at 4 weeks (P<0.05; Tables 4 and 5, Figures 2 and 3). The IPSS storage sub-score was not significantly altered in any subgroup at 4 weeks compared with at baseline. The IPSS voiding sub-score was significantly decreased in the NP subgroup and decreased in the NBC subgroup but not in the nocturia due to both causes subgroups (Tables 4 and 5, Figures 2 and 3). Qmax and PVR were not changed at 12 weeks.
Table 1.
Baseline characteristics in International Prostate Symptom Score (IPSS) and uroflowmetry parameters, 3-day voiding diary (VD) of Groups Aa and Bb
| Characteristic | Group A | Group B | P-value |
|---|---|---|---|
| Patients | |||
| N | 209 | 196 | |
| Age, years | 66.6±5.4 | 64.6±4.4 | 0.56 |
| BMI, kg/m2 | 23.5±2.5 | 22.8±1.9 | 0.72 |
| IPSS | |||
| Total | 18.0±5.3 | 16.5±4.5 | 0.38 |
| Voiding symptoms | 9.0±3.2 | 8.4±3.5 | 0.64 |
| Storage symptoms | 9.0±2.7 | 8.1±2.5 | 0.73 |
| Nocturia | 2.9±0.5 | 2.8±1.1 | 0.86 |
| OABSS | 11.4±5.2 | 12.8±5.7 | 0.23 |
| Uroflowmetry | |||
| Qmax, mL/second | 13.5±4.7 | 14.9±5.9 | 0.17 |
| PVR, mL | 33.6±22.7 | 32.9±20.2 | 0.69 |
| 3-day VD | |||
| Nocturia episodes in 3 days, n | 8.7±3.2 | 8.6±2.9 | 0.93 |
| Nocturnal urine volume, mL | 666.0±132.9 | 710.0±152.1 | 0.14 |
| Nocturnal index | 1.4±0.3 | 1.6±0.7 | 0.37 |
| Urgency episodes in 3 days, n | 5.6±2.9 | 5.9±1.9 | 0.54 |
| NBC index | 0.5±0.1 | 0.5±0.2 | 0.93 |
Notes: Values are mean ± standard deviation.
Alpha-blocker for 4 weeks, followed by alpha-blocker plus an anticholinergic agent for 4 weeks, then, lastly, an alpha-blocker plus an antidiuretic agent for 4 weeks
alpha-blocker for 4 weeks, followed by alpha-blocker plus an antidiuretic agent for 4 weeks, then, lastly, an alpha-blocker plus an anticholinergic agent for 4 weeks.
Abbreviations: BMI, body-mass index; NBC, nocturnal bladder capacity; OABSS, Overactive Bladder Symptom Score; PVR, post-void residual urine; Qmax, maximum urinary flow rate; n, number.
Table 2.
Baseline characteristics of International Prostate Symptom Score (IPSS) and uroflowmetry parameters, 3-day voiding diary (VD) of Group Aa
| Characteristic | NP | Decreased NBC | Nocturia due to both causes | P-value |
|---|---|---|---|---|
| Patients | ||||
| N | 83 | 51 | 75 | |
| Age, years | 62.3±5.7 | 63.7±5.1 | 65.1±7.5 | NS |
| BMI, kg/m2 | 23.8±2.3 | 25.3±1.5 | 22.1±3.5 | NS |
| IPSS | ||||
| Total | 16.7±6.5 | 20.2±5.7 | 18.2±7.4 | NS |
| Voiding symptoms | 9.3±3.7 | 8.3±3.8 | 9.3±4.9 | NS |
| Storage symptoms | 7.4±2.5 | 11.9±2.5* | 8.9±3.2 | <0.05 |
| Nocturia | 2.7±0.6 | 3.0±1.3 | 3.2±1.3 | NS |
| OABSS | 6.8±3.5* | 15.5±7.2 | 13.8±5.6 | <0.05 |
| Uroflowmetry | ||||
| Qmax, mL/second | 13.4±5.1 | 9.9±2.9 | 16.2±8.7 | NS |
| PVR, mL | 35.2±24.5 | 42.5±13.2* | 25.9±20.4 | <0.05 |
| 3-day VD | ||||
| Nocturia episodes in 3 days, n | 8.6±3.1 | 9.7±4.4 | 8.2±3.7 | NS |
| Nocturnal urine volume (mL) | 768.0±126.5* | 539.0±104.9 | 642.0±96.2 | <0.05 |
| Nocturnal index | 1.5±0.4 | 1.2±0.6* | 1.7±0.4 | <0.05 |
| Urgency episodes in 3 days, n | 1.2±0.3* | 9.8±3.5 | 7.7±4.2 | <0.05 |
| NBC index | −0.3±0.1* | 1.4±0.5 | 0.8±0.3 | <0.05 |
Notes: Values are mean ± standard deviation
P<0.05.
Alpha-blocker for 4 weeks, followed by alpha-blocker plus an anticholinergic agent for 4 weeks, then, lastly, an alpha-blocker plus an antidiuretic agent for 4 weeks.
Abbreviations: BMI, body-mass index; NBC, nocturnal bladder capacity; NP, nocturnal polyuria; NS, nonsignificant; OABSS, Overactive Bladder Symptom Score; PVR, post-void residual urine; Qmax, maximum urinary flow rate.
Table 3.
Baseline characteristics of International Prostate Symptom Score (IPSS) and uroflowmetry parameters, 3-day voiding diary (VD) of Group Ba
| Characteristic | NP | Decreased NBC | Nocturia due to both causes | P-value |
|---|---|---|---|---|
| Patients | ||||
| N | 76 | 42 | 78 | |
| Age, years | 65.2±4.3 | 62.5±3.2 | 65.3±6.7 | NS |
| BMI, kg/m2 | 24.5±1.3 | 22.4±1.7 | 21.4±2.4 | NS |
| IPSS | ||||
| Total | 14.5±6.5 | 18.2±5.7 | 17.7±7.4 | NS |
| Voiding symptoms | 8.2±2.6 | 7.5±2.8 | 9.1±4.5 | NS |
| Storage symptoms | 6.3±2.5 | 10.7±3.3* | 8.6±2.8 | <0.05 |
| Nocturia | 2.5±0.7 | 3.2±1.1 | 3.1±1.6 | NS |
| OABSS | 7.2±4.1* | 17.2±6.3 | 16.1±6.3 | <0.05 |
| Uroflowmetry | ||||
| Qmax, mL/second | 15.3±5.4 | 9.2±2.2* | 17.8±10.8 | <0.05 |
| PVR, mL | 33.8±22.3 | 37.2±11.1 | 29.8±22.5 | NS |
| 3-day VD | ||||
| Nocturia episodes in 3 days, n | 8.3±3.5 | 9.2±5.1 | 8.6±2.9 | NS |
| Nocturnal urine volume (mL) | 853.0±150.3* | 562.0±93.7 | 651.0±95.8 | <0.05 |
| Nocturnal index | 1.6±0.2 | 1.3±0.5* | 1.8±0.7 | <0.05 |
| Urgency episodes in 3 days, n | 1.4±0.5* | 9.6±4.2 | 8.3±3.4 | <0.05 |
| NBC index | −0.2±0.1* | 1.3±0.5 | 0.9±0.2 | <0.05 |
Notes: Values are mean ± standard deviation
P<0.05.
Alpha-blocker for 4 weeks, followed by alpha-blocker plus an antidiuretic agent for 4 weeks, then, lastly, an alpha-blocker plus an anticholinergic agent for 4 weeks.
Abbreviations: BMI, body-mass index; NBC, nocturnal bladder capacity; NP, nocturnal polyuria; NS, nonsignificant; OABSS, Overactive Bladder Symptom Score; PVR, post-void residual urine; Qmax, maximum urinary flow rate.
Table 4.
Changes in the International Prostate Symptom Score (IPSS) and uroflowmetry parameters, 3-day voiding diary (VD) after treatment in Group Aa
| Characteristic/parameter/score | Baseline | 4 weeks (alpha-blocker) | 8 weeks (alpha-blocker + anticholinergic agent) | 12 weeks (alpha-blocker + antidiuretic agent) |
|---|---|---|---|---|
| NP (n=83) | ||||
| IPSS | ||||
| Total | 16.7±6.5 | 14.2±3.2* | 13.9±4.3 | 11.2±3.2‡ |
| Voiding symptoms | 9.3±3.7 | 7.7±4.2* | 7.8±3.1 | 6.4±2.9 |
| Storage symptoms | 7.4±2.5 | 6.5±2.5 | 6.1±3.3 | 4.8±1.5‡ |
| Nocturia | 2.7±0.6 | 2.3±0.2* | 2.4±0.6 | 1.8±0.1‡ |
| OABSS | 6.8±3.5 | 6.1±2.7 | 5.5±2.9 | 5.7±2.2 |
| Uroflowmetry | ||||
| Qmax, mL/second | 13.4±5.1 | 14.7±7.2 | 14.9±6.7 | 15.3±4.2‡ |
| PVR, mL | 35.2±24.5 | 32.1±22.3 | 33.5±21.7 | 29.1±15.3 |
| 3-day VD | ||||
| Nocturia episodes in 3 days, n | 8.6±3.1 | 7.2±2.3* | 8.0±3.7 | 6.2±2.3‡ |
| Nocturnal urine volume (mL) | 768.0±126.5 | 732.0±103.1 | 751.0±117.2 | 526.0±96.2‡ |
| Nocturnal index | 1.5±0.4 | 1.4±0.2 | 1.4±0.5 | 1.1±0.1‡ |
| Urgency episodes in 3 days, n | 1.2±0.3 | 1.0±0.4 | 1.1±0.3 | 1.2±0.2 |
| NBC index | −0.3±0.1 | −0.3±0.2 | −0.2±0.1 | −0.3±0.1 |
| Decreased NBC (n=51) | ||||
| IPSS | ||||
| Total | 20.2±5.7 | 18.4±7.3* | 16.2±5.1‡ | 18.1±9.5 |
| Voiding symptoms | 8.3±3.8 | 7.6±1.8* | 7.6±1.7 | 7.9±3.3 |
| Storage symptoms | 11.9±2.5 | 10.8±2.5 | 8.6±1.5‡ | 10.2±2.5 |
| Nocturia | 3.0±1.3 | 2.9±1.1 | 2.2±0.3‡ | 2.8±1.2 |
| OABSS | 15.5±7.2 | 14.7±6.3 | 12.9±6.7‡ | 14.2±5.7 |
| Uroflowmetry | ||||
| Qmax, mL/second | 9.9±2.9 | 12.4±3.5 | 15.3±2.9 | 12.2±1.7 |
| PVR, mL | 42.5±13.2 | 36.2±11.1 | 38.5±10.1 | 40.4±11.8 |
| 3-day VD | ||||
| Nocturia episodes in 3 days, n | 9.7±4.4 | 8.2±2.9* | 6.3±2.7‡ | 8.1±3.4 |
| Nocturnal urine volume (mL) | 539.0±104.9 | 568.0±86.2 | 553.0±97.9 | 543.0±85.1 |
| Nocturnal index | 1.2±0.6 | 1.2±0.2 | 1.3±0.5 | 1.2±0.1 |
| Urgency episodes in 3 days, n | 9.8±3.5 | 9.7±4.1 | 7.2±2.6‡ | 8.8±3.4 |
| NBC index | 1.4±0.5 | 1.3±0.3 | 0.9±0.3‡ | 1.4±0.2 |
| Nocturia due to both causes (n=75) | ||||
| IPSS | ||||
| Total | 18.2±7.4 | 16.6±6.1* | 17.1±7.4 | 16.2±3.6 |
| Voiding symptoms | 9.3±4.9 | 8.9±4.9 | 9.9±4.9 | 10.3±4.3 |
| Storage symptoms | 8.9±3.2 | 7.7±3.2 | 5.9±3.2‡ | 7.2±3.2 |
| Nocturia | 3.2±1.3 | 2.5±1.1* | 2.2±0.9‡ | 2.2±1.4 |
| OABSS | 13.8±5.6 | 13.9±5.2 | 9.7±3.2‡ | 10.2±5.3‡ |
| Uroflowmetry | ||||
| Qmax, mL/second | 16.2±8.7 | 18.8±12.7 | 19.4±12.3 | 22.7±40.8 |
| PVR, mL | 25.9±20.4 | 22.9±21.3 | 23.5±18.1 | 18.5±16.2 |
| 3-day VD | ||||
| Nocturia episodes in 3 days, n | 8.2±3.7 | 7.2±2.9* | 6.3±2.7‡ | 8.1±3.4 |
| Nocturnal urine volume (mL) | 642.0±96.2 | 595.0±78.8 | 601.0±94.2 | 543.0±85.1‡ |
| Nocturnal index | 1.7±0.4 | 1.6±0.3 | 1.4±0.7 | 1.3±0.2‡ |
| Urgency episodes in 3 days, n | 7.7±4.2 | 7.2±3.9 | 5.4±2.3‡ | 7.5±3.3 |
| NBC index | 0.8±0.3 | 0.8±0.3 | 0.5±0.1‡ | 0.9±0.2 |
Notes: Values are mean ± standard deviation
P<0.05 changes from baseline
P<0.05 changes from 4 weeks.
Alpha-blocker for 4 weeks, followed by alpha-blocker plus an anticholinergic agent for 4 weeks, then, lastly, an alpha-blocker plus an antidiuretic agent for 4 weeks.
Abbreviations: NBC, nocturnal bladder capacity; NP, nocturnal polyuria; OABSS, Overactive Bladder Symptom Score; PVR, post-void residual urine; Qmax, maximum urinary flow rate; VD, voiding diary.
Table 5.
Changes in the International Prostate Symptom Score (IPSS) and uroflowmetry parameters, 3-day VD after treatment in Group Ba
| Characteristic/parameter/score | Baseline | 4 weeks (alpha-blocker) | 8 weeks (alpha-blocker + antidiuretic agent) | 12 weeks (alpha-blocker + anticholinergic agent) |
|---|---|---|---|---|
| NP (n=76) | ||||
| IPSS | ||||
| Total | 14.5±6.5 | 12.1±3.2* | 11.5±2.7‡ | 13.7±5.2 |
| Voiding symptoms | 8.2±2.6 | 6.7±4.4* | 7.2±3.3 | 7.9±4.1 |
| Storage symptoms | 6.3±2.5 | 6.4±3.1 | 4.3±1.5‡ | 5.8±2.6 |
| Nocturia | 2.5±0.7 | 2.1±0.4 | 1.7±0.2‡ | 2.5±0.7 |
| OABSS | 7.2±4.1 | 6.8±2.7 | 6.5±2.4 | 7.0±3.5 |
| Uroflowmetry | ||||
| Qmax, mL/second | 15.3±5.4 | 15.9±5.2 | 14.6±5.1 | 12.5±4.2 |
| PVR, mL | 33.8±22.3 | 31.5±15.2 | 29.7±12.5 | 33.5±21.7 |
| 3-day VD | ||||
| Nocturia episodes in 3 days, n | 8.3±3.5 | 6.4±1.7* | 5.8±2.6‡ | 7.0±4.5 |
| Nocturnal urine volume (mL) | 853.0±150.3 | 798.0±92.4 | 559.0±93.1‡ | 815.0±106.5 |
| Nocturnal index | 1.6±0.2 | 1.5±0.4 | 1.0±0.2‡ | 1.4±0.7 |
| Urgency episodes in 3 days, n | 1.4±0.5 | 1.3±0.8 | 1.1±0.2 | 1.4±0.4 |
| NBC index | −0.2±0.1 | −0.2±0.2 | −0.2±0.1 | −0.1±0.1 |
| Decreased NBC (n=42) | ||||
| IPSS | ||||
| Total | 18.2±5.7 | 16.2±5.4* | 16.1±9.5 | 13.2±5.1‡ |
| Voiding symptoms | 7.5±2.8 | 6.5±1.8* | 6.4±3.3 | 6.9±1.7 |
| Storage symptoms | 10.7±3.3 | 9.7±2.5 | 9.7±2.8 | 6.3±1.2‡ |
| Nocturia | 3.2±1.1 | 2.8±1.3 | 2.7±0.9 | 2.0±0.6‡ |
| OABSS | 17.2±6.3 | 15.8±7.1 | 15.5±6.7 | 13.1±7.4‡ |
| Uroflowmetry | ||||
| Qmax, mL/second | 9.2±2.2 | 10.8±2.5 | 15.3±2.7 | 12.6±2.2 |
| PVR, mL | 37.2±11.1 | 30.3±13.2 | 23.1±10.7 | 32.1±13.2 |
| 3-day VD | ||||
| Nocturia episodes in 3 days, n | 9.2±5.1 | 7.3±2.5* | 7.4±2.4 | 6.1±2.5‡ |
| Nocturnal urine volume (mL) | 562.0±93.7 | 571.0±89.1 | 533.0±82.1 | 623.0±121.9 |
| Nocturnal index | 1.3±0.5 | 1.3±0.2 | 1.1±0.1 | 1.2±0.3 |
| Urgency episodes in 3 days, n | 9.6±4.2 | 9.7±3.5 | 9.3±3.7 | 7.1±5.3‡ |
| NBC index | 1.3±0.5 | 1.2±0.6 | 1.3±0.3 | 0.5±0.1‡ |
| Nocturia due to both causes (n=78) | ||||
| IPSS | ||||
| Total | 17.7±7.4 | 14.3±5.9* | 13.0±3.1 | 12.4±7.4‡ |
| Voiding symptoms | 9.1±4.5 | 6.9±2.5 | 7.1±3.9 | 6.2±4.9 |
| Storage symptoms | 8.6±2.8 | 7.4±3.2 | 5.9±2.5 | 5.2±3.2‡ |
| Nocturia | 3.1±1.6 | 2.3±1.2* | 2.2±1.7 | 2.3±0.5 |
| OABSS | 16.1±6.3 | 14.8±7.5 | 13.2±6.1‡ | 12.4±4.8‡ |
| Uroflowmetry | ||||
| Qmax mL/sec | 17.8±10.8 | 20.1±13.4 | 20.5±10.6 | 18.2±10.9 |
| PVR, mL | 29.8±22.5 | 25.7±20.7 | 21.5±18.3 | 25.5±17.3 |
| 3-day VD | ||||
| Nocturia episodes in 3 days, n | 8.6±2.9 | 7.0±2.1* | 6.8±3.3 | 6.9±2.5 |
| Nocturnal urine volume (mL) | 651.0±95.8 | 623.0±89.8 | 521.0±64.7‡ | 631.0±92.3 |
| Nocturnal index | 1.8±0.7 | 1.5±0.6 | 1.4±0.3 | 1.4±0.8 |
| Urgency episodes in 3 days, n | 8.3±3.4 | 7.9±2.8 | 8.1±2.9 | 6.5±3.2‡ |
| NBC index | 0.9±0.2 | 0.9±0.5 | 0.8±0.5 | 0.8±0.1 |
Notes: Values are mean ± standard deviation;
P<0.05 changes from baseline.
P<0.05 changes from 4-weeks.
Alpha-blocker for 4 weeks, followed by alpha-blocker plus an antidiuretic agent for 4 weeks, then, lastly, an alpha-blocker plus an anticholinergic agent for 4 weeks.
Abbreviations: NBC, nocturnal bladder capacity; NP, nocturnal polyuria; OABSS, Overactive Bladder Symptom Score; PVR, post-void residual urine; Qmax, maximum urinary flow rate; VD, voiding diary.
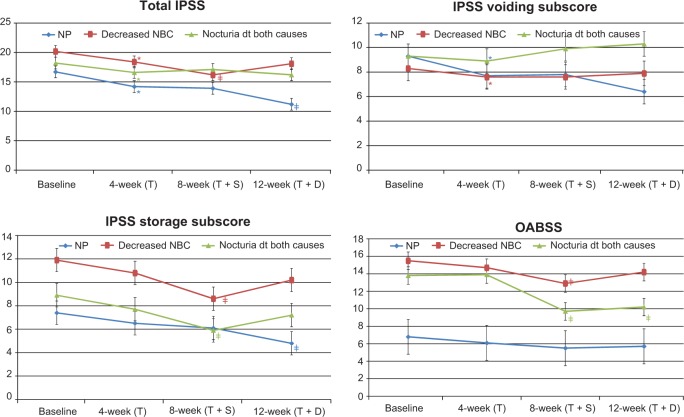
Figure 2.
Changes in International Prostate Symptom Score (IPSS), IPSS sub-scores and Overactive Bladder Symptom Score (OABSS) after treatment in Group A.
Notes: *P<0.05 changes from baseline; ‡P<0.05 changes from 4 weeks.
Abbreviations: dt, due to; NBC, nocturnal bladder capacity; NP, nocturnal polyuria; T, tamsulosin; T + D, tamsulosin + desmopressin; T + S, tamsulosin + solifenacin.
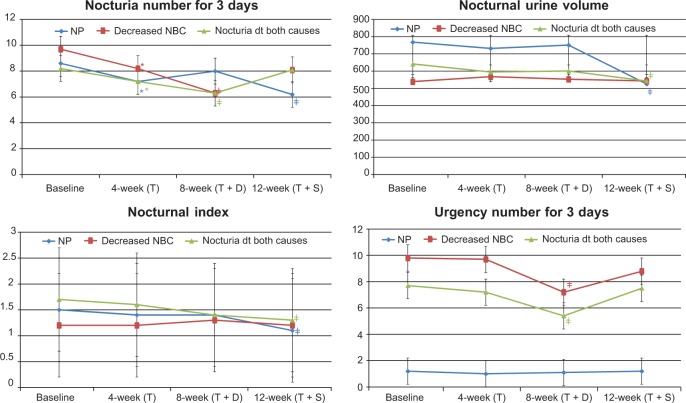
Figure 3.
Changes in 3-day voiding diary after treatment in Group A.
Notes: *P<0.05 changes from baseline; ‡P<0.05 changes from 4 weeks.
Abbreviations: dt, due to; NBC, nocturnal bladder capacity; NP, nocturnal polyuria; T, tamsulosin; T + D, tamsulosin + desmopressin; T + S, tamsulosin + solifenacin.
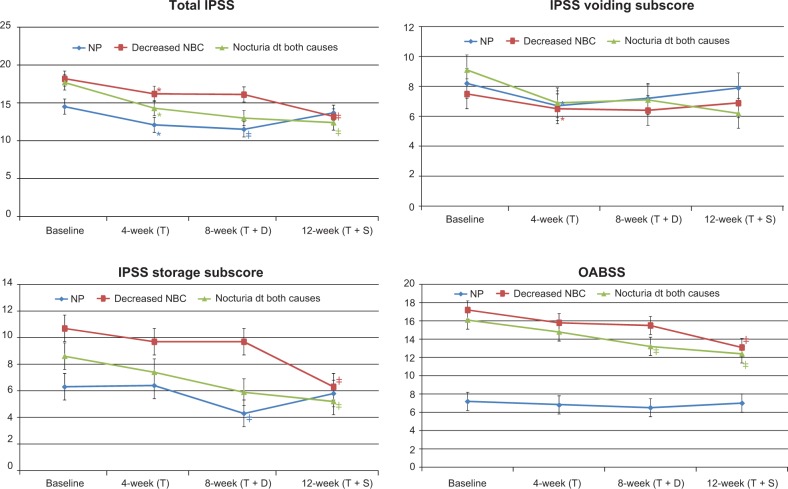
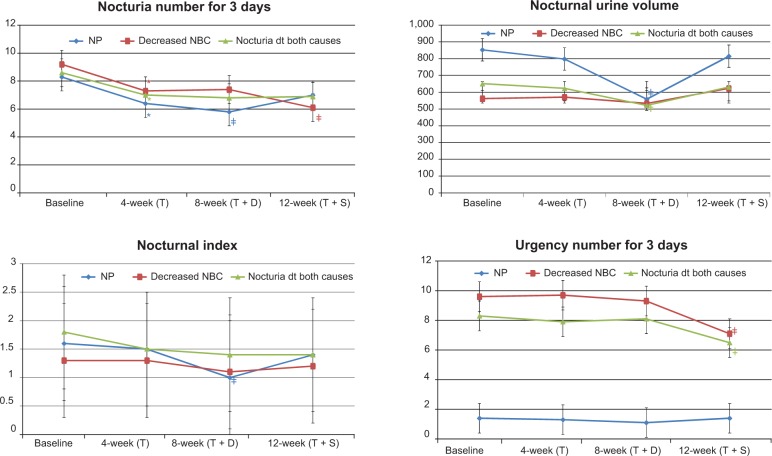
In the NP subgroup of Group A, the number of episodes of nocturia in 3 days, nocturnal urine volume, and nocturnal index were significantly decreased at 12 weeks compared with at 4 weeks (P<0.05; Table 4 and Figure 4). In Group B, nocturnal urine volume and NP index were also decreased significantly at 8 weeks compared with at 4 weeks (P<0.05; Table 5 and Figure 5).
Figure 4.
Changes in International Prostate Symptom Score (IPSS), IPSS sub-scores, and Overactive Bladder Symptom Score (OABSS) after treatment in Group B.
Notes: *P<0.05 changes from baseline; ‡P<0.05 changes from 4 weeks.
Abbreviations: dt, due to; NBC, nocturnal bladder capacity; NP, nocturnal polyuria; T, tamsulosin; T + D, tamsulosin + desmopressin; T + S, tamsulosin + solifenacin.
Figure 5.
Changes in 3-day voiding diary after treatment in Group B.
Notes: *P<0.05 changes from baseline; ‡P<0.05 changes from 4 weeks.
Abbreviations: dt, due to; NBC, nocturnal bladder capacity; NP, nocturnal polyuria; T, tamsulosin; T + D, tamsulosin + desmopressin; T + S, tamsulosin + solifenacin.
In the decreased NBC subgroup of Group A, IPSS storage sub-score, OABSS, number of episodes of nocturia in 3 days, number of episodes of urgency in 3 days, and NBC index were significantly decreased at 8 weeks compared with at 4 weeks (P<0.05; Table 4 and Figures 2 and 4). Also in Group B, IPSS storage sub-score, OABSS, number of episodes of nocturia in 3 days, number of episodes of urgency in 3 days, and NBC index were decreased significantly at 12 weeks compared with at 4 weeks (P<0.05; Table 5 and Figures 3 and 5).
In the nocturia due to both causes subgroup of Group A, IPSS storage sub-score, OABSS, and number of episodes of urgency in 3 days were significantly decreased at 8 weeks compared with at 4 and 12 weeks, while nocturnal urine volume and OABSS were significantly decreased at 12 weeks compared with at 4 weeks (P<0.05; Table 4 and Figures 2 and 4). In Group B, IPSS storage sub-score, OABSS, and number of episodes of urgency in 3 days were decreased significantly at 12 weeks compared with at 4 weeks, and nocturnal urine volume and OABSS were significantly decreased at 8 weeks compared with at 4 weeks (P<0.05; Table 5 and Figures 3 and 5).
Discussion
LUTS include OAB symptoms like frequency, urgency, and incontinence. OAB symptoms are generally more bothersome and represent an important target in the management of BPH. Incontinence frequency, urgency, and urge have been attributed to detrusor overactivity, which reportedly occurs in 40%–70% of patients with BOO.15 Alpha-blockers, which promote relaxation of the bladder neck and prostate smooth muscle, have had limited success in the treatment of OAB-related symptoms.16 Kaplan et al demonstrated that relative to subjects receiving placebo, men receiving extended-release tolterodine and tamsulosin had significant improvements in the total IPSS and quality-of-life scores, number of urgency episodes and urge incontinence episodes.6 Lee et al reported benefits for men with OAB symptoms from an initial combination treatment of an alpha-blocker with anticholinergics, without increased risk of voiding difficulty and acute urinary retention.17
In our study, the combination treatment of an alpha-blocker plus an anticholinergic agent proved beneficial for the decreased NBC subgroup of Groups A and B. The IPSS storage sub-score, OABSS, number of episodes of nocturia in 3 days, number of episodes of urgency in 3 days, and NBC index were significantly decreased in these subjects.
Nocturia cannot be explained based on BPH alone, but can be consecutive to polyuria, diabetes mellitus, neurogenic bladder, cardiac failure, polydipsia, reduced bladder capacity, insomnia, or psychiatric problems. To reduce nocturia, actions can be directed at BOO, bladder sensitivity by anticholinergics, sleepiness by hypnotic drugs, or urinary volume by antidiuretics.18 The serum concentration of the antidiuretic agent vasopressin increases during the night, which decreases urine secretion. Secretion of this hormone diminishes with age, when the renal response to antidiuretic agents decreases and the reduction of the total amount of nephrons limit the renal response to the hormone.18 Antidiuretic agents reduce nocturnal diuresis and the number of nocturnal voids, and increase the time between going to bed and the first nocturnal void. Several clinical trials investigating the benefits of desmopressin therapy for patients with nocturia have been completed.10–12 In our study, the combination treatment of an alpha-blocker with an antidiuretic agent provided benefits for the NP subgroup of Groups A and B. The number of episodes of nocturia in 3 days, nocturnal urine volume, and nocturnal index were significantly decreased.
Objective measurement of subjective LUTS has proven to be a clinical challenge. A patient-completed 3-day VD is commonly used in clinical trials as a primary tool for measuring these symptoms. Frequency-volume chart records the volumes voided as well as the time of each micturition, day and night, for at least 24 hours, while a VD records the times of micturitions and voided volumes, incontinence episodes, pad usage, and other information, such as the degree of urgency and degree of incontinence.19 These measures are noninvasive, inexpensive, and accurate. As the 3-day VD is usually recorded during normal daily activities, it can also provide important information on a patient’s voiding problem.20 The 3-day duration seems to be the most commonly used form to ensure the accuracy of the VD, as well as minimizing the burden of recording.21 Combined with other clinical findings and diagnostic tools, the 3-day VD might provide additional information.
Our study did not show a benefit with the medication order of anticholinergic agent or antidiuretic agent as add-on therapy for nocturia in men previously treated with an alpha-blocker for LUTS. An anticholinergic agent or an antidiuretic agent added to alpha-blocker therapy, according to voiding disorder (nocturnal polyuria, decreased NBC, or nocturia by both causes subgroups), was found to provide benefits to refractory nocturia in men previously treated with an alpha-blocker for LUTS.
There are some limitations to our study that should be considered when interpreting the results. First, 5-alpha reductase inhibitors were not considered as a medication for LUTS. A 5-alpha reductase inhibitor along with an anticholinergic agent or antidiuretic agent added to an alpha-blocker could be helpful to patients with refractory nocturia. Second, the period of treatment for each sequence was limited to 4 weeks. A longer period for each sequence could have provided more information and should be assessed. Third, the collection of information was based on a self-reported VD, so reporting bias was inevitable. A monitoring system should be considered in subsequent studies to bolster the results.
Notwithstanding these limitations, as far as we are aware, the present study is the first to report that adding an anticholinergic or antidiuretic agent to an alpha-blocker provides better results in terms of improving LUTS, especially nocturia, than an alpha-blocker alone.
Acknowledgments
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (HI13C0104). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
Study concept and design: Jong Kwan Park and Yu Seob Shin; organization and data management: Yu Seob Shin, Chen Zhao, Li Tao Zhang, Young Gon Kim, and Jong Kwan Park; intervention design: Jong Kwan Park, Yu Seob Shin; study physicians: Jong Kwan Park and Yu Seob Shin; statistical analysis: Jong Kwan Park and Yu Seob Shin; analysis and interpretation of data: Jong Kwan Park and Yu Seob Shin, Chen Zhao, and Li Tao Zhang. All authors drafted or revised, commented on, and approved the final version of the manuscript for publication.
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Blake-James BT, Rashidian A, Ikeda Y, Emberton M. The role of anticholinergics in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a systematic review and meta-analysis. BJU Int. 2007;99(1):85–96. doi: 10.1111/j.1464-410X.2006.06574.x. [DOI] [PubMed] [Google Scholar]
- 2.Chapple CR, Roehrborn CG. A shifted paradigm for the further understanding, evaluation, and treatment of lower urinary tract symptoms in men: focus on the bladder. Eur Urol. 2006;49(4):651–658. doi: 10.1016/j.eururo.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 3.Irwin DE, Milsom I, Hunskaar S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol. 2006;50(6):1306–1314. doi: 10.1016/j.eururo.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 4.Athanasopoulos A, Gyftopoulos K, Giannitsas K, Fisfis J, Perimenis P, Barbalias G. Combination treatment with an alpha-blocker plus an anticholinergic for bladder outlet obstruction: a prospective, randomized, controlled study. J Urol. 2003;169(6):2253–2256. doi: 10.1097/01.ju.0000067541.73285.eb. [DOI] [PubMed] [Google Scholar]
- 5.Lee KS, Choo MS, Kim DY, et al. Combination treatment with propiverine hydrochloride plus doxazosin controlled release gastrointestinal therapeutic system formulation for overactive bladder and coexisting benign prostatic obstruction: a prospective, randomized, controlled multicenter study. J Urol. 2005;174(4 Pt 1):1334–1338. doi: 10.1097/01.ju.0000173630.94559.fd. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan SA, Roehrborn CG, Rovner ES, Carlsson M, Bavendam T, Guan Z. Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial. JAMA. 2006;296(19):2319–2328. doi: 10.1001/jama.296.19.2319. [DOI] [PubMed] [Google Scholar]
- 7.Ali A, Snape J. Nocturia in older people: a review of causes, consequences, assessment and management. Int J Clin Pract. 2004;58(4):366–373. doi: 10.1111/j.1368-5031.2004.00086.x. [DOI] [PubMed] [Google Scholar]
- 8.Yoshimura K, Ohara H, Ichioka K, et al. Nocturia and benign prostatic hyperplasia. Urology. 2003;61(4):786–790. doi: 10.1016/s0090-4295(02)02444-5. [DOI] [PubMed] [Google Scholar]
- 9.Chang SC, Lin AT, Chen KK, Chang LS. Multifactorial nature of male nocturia. Urology. 2006;67(3):541–544. doi: 10.1016/j.urology.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 10.Mattiasson A, Abrams P, Van Kerrebroeck P, Walter S, Weiss J. Efficacy of desmopressin in the treatment of nocturia: a double-blind placebo-controlled study in men. BJU Int. 2002;89(9):855–862. doi: 10.1046/j.1464-410x.2002.02791.x. [DOI] [PubMed] [Google Scholar]
- 11.Lose G, Mattiasson A, Walter S, et al. Clinical experiences with desmopressin for long-term treatment of nocturia. J Urol. 2004;172(3):1021–1025. doi: 10.1097/01.ju.0000136203.76320.f6. [DOI] [PubMed] [Google Scholar]
- 12.Weiss JP, Zinner NR, Klein BM, Nørgaard JP. Desmopressin orally disintegrating tablet effectively reduces nocturia: results of a randomized, double-blind, placebo-controlled trial. Neurourol Urodyn. 2012;31(4):441–447. doi: 10.1002/nau.22243. [DOI] [PubMed] [Google Scholar]
- 13.Kirby RS. A randomized, double-blind crossover study of tamsulosin and controlled-release doxazosin in patients with benign prostatic hyperplasia. BJU Int. 2003;91:41–44. doi: 10.1046/j.1464-410x.2003.03077.x. [DOI] [PubMed] [Google Scholar]
- 14.Homma Y, Yoshida M, Seki N, Yokoyama O, et al. Symptom assessment tool for overactive bladder syndrome – overactive bladder symptom score. Urology. 2006;68:318–323. doi: 10.1016/j.urology.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 15.Hyman MJ, Groutz A, Blaivas JG. Detrusor instability in men: correlation of lower urinary tract symptoms with urodynamic findings. J Urol. 2001;166(2):550–552. doi: 10.1016/s0022-5347(05)65982-4. [DOI] [PubMed] [Google Scholar]
- 16.Lee JY, Kim HW, Lee SJ, Koh JS, Suh HJ, Chancellor MB. Comparison of doxazosin with or without tolterodine in men with symptomatic bladder outlet obstruction and an overactive bladder. BJU Int. 2004;94(6):817–820. doi: 10.1111/j.1464-410X.2004.05039.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee SH, Chung BH, Kim SJ, Kim JH, Kim JC, Lee JY. Initial combined treatment with anticholinergics and a-blockers for men with lower urinary tract symptoms related to BPH and overactive bladder: a prospective, randomized, multi-center, double-blind, placebo-controlled study. Prostate Cancer Prostatic Dis. 2011;14(4):320–325. doi: 10.1038/pcan.2011.22. [DOI] [PubMed] [Google Scholar]
- 18.Asplund R, Aberg H. Diurnal variation in the levels of antidiuretic hormone in the elderly. J Intern Med. 1991;229(2):131–134. doi: 10.1111/j.1365-2796.1991.tb00320.x. [DOI] [PubMed] [Google Scholar]
- 19.Abrams P, Klevmark B. Frequency volume charts: an indispensable part of lower urinary tract assessment. Scand J Urol Nephrol Suppl. 1996;179:47–53. [PubMed] [Google Scholar]
- 20.Matthiesen TB, Rittig S, Mortensen JT, Djurhuus JC. Nocturia and polyuria in men referred with lower urinary tract symptoms, assessed using a 7-day frequency-volume chart. BJU Int. 1999;83(9):1017–1022. doi: 10.1046/j.1464-410x.1999.00090.x. [DOI] [PubMed] [Google Scholar]
- 21.Tincello DG, Williams KS, Joshi M, Assassa RP, Abrams KR. Urinary diaries: a comparison of data collected for three days versus seven days. Obstet Gynecol. 2007;109(2 Pt 1):277–280. doi: 10.1097/01.AOG.0000252832.21986.c8. [DOI] [PubMed] [Google Scholar]