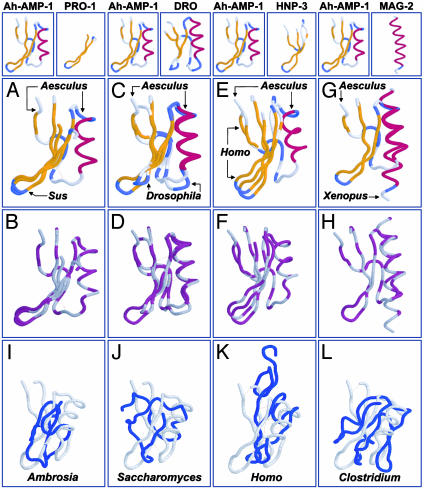
Fig. 2.
(A-H) Conservation of 3D structure among disulfide-stabilized antimicrobial peptides. Comparisons (peptide name, PDB ID code, source genus, common name; rmsd) are between Ah-AMP-1 (1BK8, Aesculus, horse chestnut tree) and protegrin-1 (PRO-1,1PG1, Sus, domestic pig; rmsd 1.2 Å; A and B); drosomycin (DRO, 1MYN, Drosophila, fruit fly; rmsd 1.2 Å; C and D); HNP-3 (1DFN, Homo, human; rmsd 3.2 Å; E and F); and magainin-2 (MAG-2, 2MAG, Xenopus, frog; rmsd 2.6 Å; G and H). A, C, E, and G use clustal secondary structure coloration (gold, β-sheet; red, α-helix; blue, turn). B, D, F, and H use the clustal polarity-2 color scheme (hydrophobic, gray; hydrophilic, purple). Oxidized cysteine residues (cystine) are colored gray, indicating hydrophobicity. Disulfide bonds are indicated as dotted yellow lines in A-H. Proteins were visualized by using protein explorer (26). (I-L) Absence of the γ-core signature in nonantimicrobial peptides. Representative comparisons are between the antimicrobial peptide Ah-AMP-1 and nonantimicrobial peptides: allergen-5 (2BBG, Ambrosia, ragweed; rmsd 6.5 Å; I); metallothionein II (1AOO, Saccharomyces, yeast; rmsd 5.3 Å; J); transforming growth factor α (3TGF, Homo, human; rmsd 4.7 Å; K); and ferredoxin (2FDN, Clostridium, bacterium; rmsd 7.4 Å; L). Nonantimicrobial peptides (blue) are shown in maximal alignment to Ah-AMP-1 (gray). Formatting is same as in A-H.