Abstract
Background
Following massive small bowel resection (SBR), a postoperative diet that is high in fat is associated with enhanced villus growth. The purpose of this study was to further elucidate the quantity and composition of enteral fat in structural and metabolic changes after SBR.
Methods
C57/Bl6 mice underwent a 50% proximal SBR. Mice were then randomized to receive a low fat diet (LFD- 12% kcal fat), medium fat diet (MFD-44% kcal fat), or high fat diet (HFD-71% kcal fat) ad lib. In a separate experiment, mice underwent 50% proximal SBR and then randomized to liquid diets of 42% kcal of fat in which the fat was composed of menhaden oil, milk fat, or olive oil. After 2 weeks, mice underwent body composition analysis and the small intestine harvested.
Results
Mice that ingested the greatest amount of enteral fat (HFD) had the highest percent lean mass. When the effects of the different kinds of enteral fat were analyzed, mice that consumed menhaden oil had the greatest percent lean mass with the greatest overall retention of preoperative weight.
Conclusions
These findings suggest that enteral fat enriched in omega-3 fatty acids may offer significant metabolic advantages for patients with short gut syndrome.
Keywords: small bowel resection, high fat diet, body composition, adaptation
Introduction
After massive small bowel resection (SBR), patients generally experience a significant weight loss with depletion of fat stores and muscle protein. This effect is due to intestinal loss and subsequent malabsorption but is also secondary to the enhanced metabolic demands resulting from the stress of surgery as well as wound healing. The goal of optimizing postoperative nutrition is to provide sufficient calories to meet these demands and prevent catabolic loss of lean body mass.
The role of diets that are high in fat in the postoperative period have been evaluated in both animal and human models. Enteral fat after small bowel resection (SBR) is associated with augmented villus growth.1-3 Additionally, we have found that a high fat diet may be beneficial in the retention of lean mass.4 However, the mechanism for high fat induced intestinal adaptation remains unknown. While it has been suggested that a low fat diet is detrimental to adaptation5, 6, it is unknown how much fat is needed to generate an augmented adaptation response. Additionally, it is unknown what kind of fat produces the optimal response after resection. While clinical studies have deemed polyunsaturated omega-3 fatty acids to be an essential component of immunonutrition7-9, there have been animal studies that have shown post surgical benefits to other types of fat including monounsaturated and saturated long chain fatty acids. 1, 4 The purpose of this study therefore was to elucidate the role for enteral fat in the structural and metabolic changes after SBR.
Methods
Animals
Male C57BL/6 mice were acquired from Jackson Laboratories (Bar Harbor, ME) and were kept on a 12-h light-dark schedule and operated on at aged 7-9 weeks. Protocols for this study were approved by the Washington University Animal Studies Committee (Protocol 20100103 and 20130308) and were in accordance with the National Institute of Health laboratory animal care and use guidelines.
Experimental Design and Diets
In the first set of experiments, all mice underwent a 50% proximal SBR. On postoperative day (POD) 1, mice were randomized into 3 separate diet groups: low fat diet (LFD- 12% kcal fat; n = 9), medium fat diet (MFD-44% kcal fat; n = 9), or high fat diet (HFD-71% kcal fat; n = 10) (Dyets Inc., Bethlahem, PA; Table 1). Despite varied percent of fat calories, all diets were isocaloric. The fat content of these diets included increasing amounts of olive oil (monounsaturated long chain fatty acids) and corn oil (omega-6 polyunsaturated fatty acids).
| Low Fat | Medium Fat | High Fat | Menhaden Oil | Milk Fat | Olive Oil | |
|---|---|---|---|---|---|---|
| Calories (kcal/ml) | 1 | 1 | 1 | 1 | 1 | 1 |
| % Kcal Fat | 12% | 44% | 71% | 42% | 42% | 42% |
| % Kcal Carbohydrates | 70% | 38% | 11% | 40% | 40% | 40% |
| % Kcal Protein | 18% | 18% | 18% | 18% | 18% | 18% |
In the second set of experiments, mice similarly underwent SBR and then randomized into 3 diet groups: menhaden oil (n = 7), olive oil (n = 9), and milk fat (n = 7)(Dyets Inc., Bethlahem, PA; Table 1). These diets are all isocaloric with equal amounts of fat calories (42% kcal fat) but with different contents of fat representing polyunsaturated long chain fatty acids (primarily omega-3), monounsaturated long chain fatty acids, and saturated long chain fatty acids, respectively.
All mice were fed with their respective diets ad lib until POD 7, in which all mice were transferred to individual metabolic cages with wire inserts that permitted collection of wasted food and feces. The quantity of food ingested and feces excreted were measured everyday until harvest. Mice were also weighed daily from POD 7 to POD 14. On POD 14, mice underwent body composition analysis and the small intestine was harvested. Isolation of enterocytes from the bowel wall involved calcium chelation and mechanical dissociation as previously described.10
Operation
Mice underwent 50% proximal SBR as we have previously illustrated.11 Briefly, mice that underwent SBR had transection of the bowel at a point 12-cm proximal to the ileal-cecal junction and also at a point 1 to 2-cm distal to the ligament of Treitz. The mesentery was ligated, and the intervening bowel was removed. Intestinal continuity was restored with an end-to-end single-layer, interrupted anastomosis using 9-0 monofilament suture. After the operation, mice were provided free access to water for the first 24 hours after surgery.
Body Composition Analysis
Mice underwent body composition analysis on POD14. Measurements were taken on awake mice with magnetic resonance imaging (MRI; EchoMRI 3-1, Echo Medical Systems) following manufacture’s instruction. The EchoMRI system creates a magnetic field around the animal and then creates contrast between tissues by releasing radio pulses, which then cause hydrogen protons to spin. The high contrast between fat and lean muscle tissue is enhanced by specific radio pulse frequencies. When the pulse is stopped, the protons then emit energy, which are then received and analyzed.
Histology
A 2-cm segment of tissue was removed from the distal portion of the resected bowel and fixed for histology in formalin during the time of operation. During harvest, a 2-cm segment was removed 12-cm proximal to the terminal ileum, adjacent to the anastomosis, and fixed in formalin.
To assess for structural adaptation, villus height and crypt depth were measured via H&E-stained histology. At least 20 well-oriented crypts and villi were counted per slide. Crypts were counted only if the crypt-villus junctions on both sides of the crypt were intact and if Paneth cells were present at the base of the crypt. Villi were counted only if the central lymphatic channel extended from the villus base to the tip and if the mucosal surface was in continuity with an intact crypt.
Real Time PCR
RNA was prepared as previously described10, from crypt and villus enterocytes and homogenized in lysis buffer (RNAqueous kit, Ambion, Austin, TX), the RNA was extracted according to kit instructions and stored at −80°C. Total RNA concentration was determined using a NanoDrop Spectrophotometer (ND-1000, NanoDrop Technologies, Wilmington, DE). CD36, ApoB, MTTP, DGAT, GLUT2, and SGLT1 primers were obtained from Life Technologies (Carlsbad, CA). β-Actin was used as the endogenous control (Applied Biosystems, Foster City, CA), and whole bowel was used as a calibrator. Real Time PCR reagents were from Applied Biosystems (Foster City, CA), and an Applied Biosystems 7500 Fast Real-Time PCR system was used (Foster City, CA).
Statistics
For most experiments, means were calculated and compared using a Student’s t-test. For comparison of weight gain over time, ANCOVA was performed (statistiXL 1.10, Nedlands, Western Australia). Values in the text are means ± SEM. Differences were considered significant at p≤0.05.
Results
HFD mice have the greatest postoperative lean mass
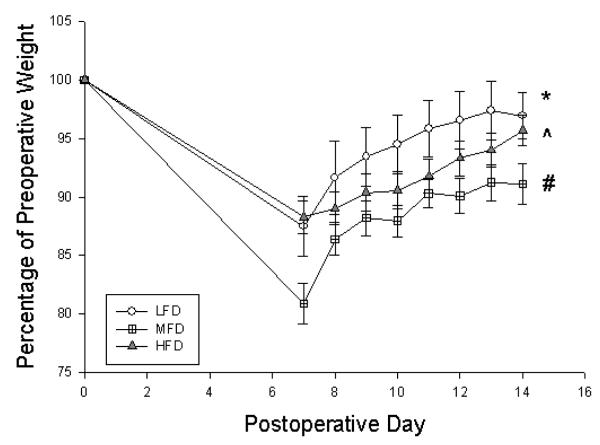
All mice experienced some degree of weight lost after surgery followed by gradual weight gain (Figure 1). LFD mice gained the most weight, followed by HFD mice although there were no differences between groups by POD14. MFD gained the least amount of weight and did not regain their preoperative weight by time of harvest.
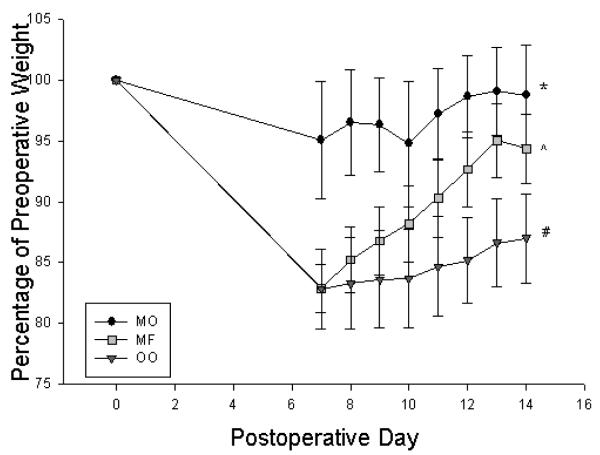
Figure 1.
Percentage of preoperative weight of mice on postoperative days 7-14 based on preoperative weight. LFD = Low Fat Diet, MFD = Medium Fat Diet, HFD = High Fat Diet. * p < 0.05 between LFD and HFD, ^ p < 0.05 between HFD and MFD, # p < 0.05 between MFD and LFD
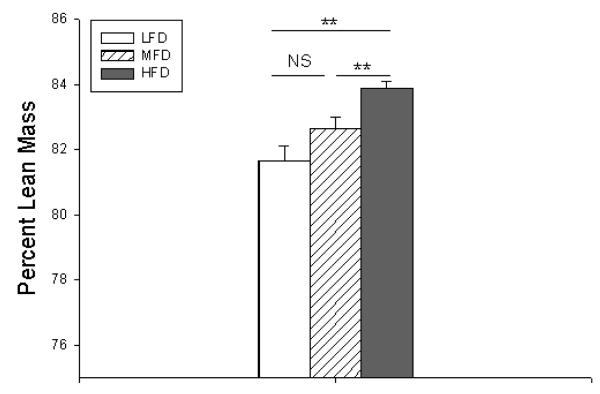
The HFD group, despite consuming the most fat, had the highest percent lean mass (Figure 2) with the lowest percent body fat (data not shown). Conversely, LFD mice had the lowest percent lean mass and highest percent body fat despite consuming the least amount of fat.
Figure 2.
Percent lean mass measured at postoperative day 14. LFD = Low Fat Diet, MFD = Medium Fat Diet, HFD = High Fat Diet. NS = No Significance, ** p < 0.005
Caloric Intake and Feces Output
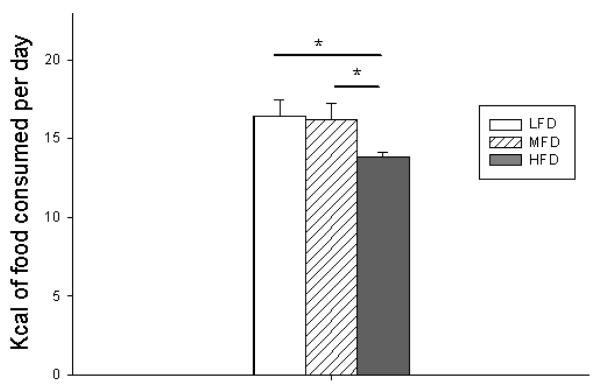
To ensure that there were no differences in food intake or feces output, all mice were individually caged with underlying wire platforms to accurately measure daily food consumption and feces excretion. As shown in Figure 3, there were no differences in food intake between LFD and MFD groups. However, HFD mice consumed fewer calories than both LFD and MFD mice. This may be due to the satiety effect of such a fat-dense diet. However, this difference did not negatively impact their weight gain or body composition. There were also no differences in fecal output (data not shown).
Figure 3.
Total kilocalories of food consumed per day. LFD = Low Fat Diet, MFD = Medium Fat Diet, HFD = High Fat Diet. * p < 0.05
All mice demonstrated adaptation
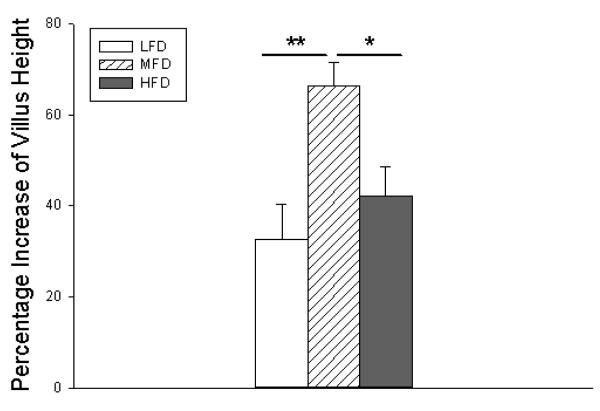
Despite having the greatest weight loss and slowest overall weight gain, MFD mice showed the greatest increase in structural adaptation as determined by the greatest degree of villus growth (Figure 4). LFD mice demonstrated normal adaptive growth despite decreased fat content. Additionally, higher fat content did not lead to increased villus height but demonstrated a similar degree of growth as LFD mice.
Figure 4.
Percent increase in villus height from intraoperative measurement to postoperative day 14. LFD = Low Fat Diet, MFD = Medium Fat Diet, HFD = High Fat Diet. * p < 0.05, ** p < 0.005
Fat and Carbohydrate Transporters
RNA was isolated from ileal villus enterocytes and used for RT-PCR of fat and carbohydrate transporters. There were no statistically significant differences in relative gene expression of the diglyceride acyltransferase (DGAT) and apolipoprotein B (ApoB) (Figure 5A). Mice fed MFD had a slight increase in microsomal triglyceride transporter protein (MTTP) expression but had a 7-fold and 3-fold increase in CD36 expression compared to LFD and HFD, respectively.
Figure 5.
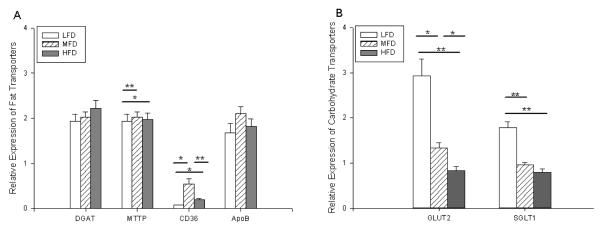
Fat (A) and carbohydrate (B) related mRNA expression of diglyceride acyltransferase (DGAT), microsomal triglyceride transporter protein (MTTP), CD36, apolipoprotein B (ApoB), glucose transporter 2 (GLUT2) and sodium glucose linked transporter (SGLT1) as determined by RT-PCR. β–Actin was used as the endogenous control, and whole bowel was used as a calibrator. LFD = Low Fat Diet, MFD = Medium Fat Diet, HFD = High Fat Diet. * p < 0.05, ** p < 0.005
Consistent with greater enteral carbohydrate consumption, the LFD group had the greatest relative gene expression of carbohydrate transporters Glucose Transporter 2 (GLUT2) and Sodium Glucose Linked Transporter (SGLT1), with nearly a 2-to-3–fold increase in GLUT2 as well as a 2– fold increase in SGLT1 over MFD and HFD, respectively (Figure 5B). Additionally, the MFD group produced nearly a 2-fold increase in GLUT2 gene expression but had no difference in SGLT1 expression compared to HFD.
Menhaden oil is associated with optimal body composition
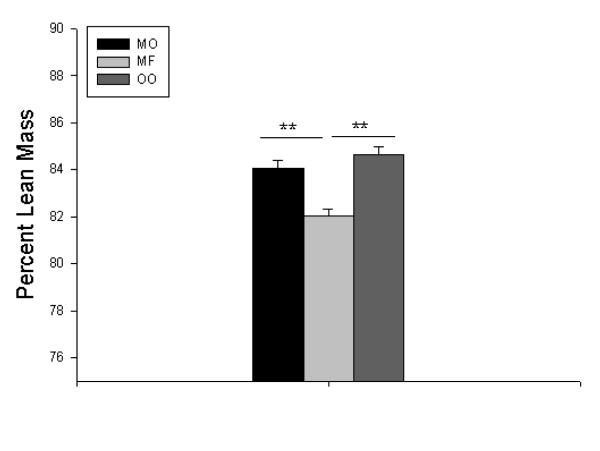
Mice who consumed menhaden oil group had greatest overall retention of preoperative weight, while the olive oil group had significant weight loss (Figure 6). In addition to having the greatest weight retention, the menhaden oil group also had the greatest percent lean mass(Figure 7) and lowest percent body fat (data not shown) compared to the saturated milk fat diet group.
Figure 6.
Percentage of preoperative weight mice on postoperative days 7-14 based on preoperative weight. MO = Menhaden Oil Diet, MF = Milk Fat Diet, OO = Olive Oil Diet. * p < 0.05 between MO and MF, ^ p < 0.05 between MF and OO, # p < 0.05 between OO and MO
Figure 7.
Percent lean mass measured at postoperative day 14. MO = Menhaden Oil Diet, MF = Milk Fat Diet, OO = Olive Oil Diet. ** p < 0.005
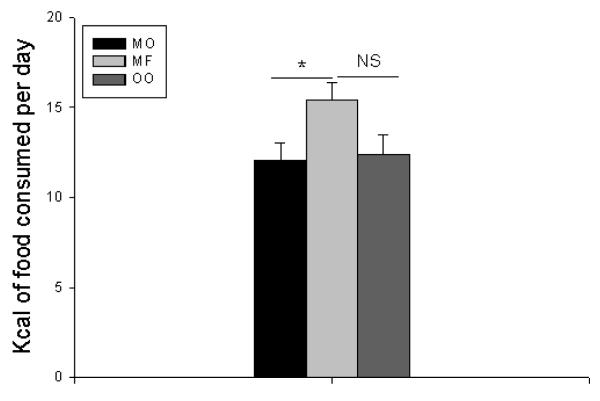
Mice consumed more calories and excreted more feces in the milk fat group than those who were fed menhaden oil, but no statistically significant difference was found when compared to the olive oil group (Figure 8). However, the milk fat group had inferior postoperative weights compared to mice that were fed menhaden oil, and so this difference may not be clinically significant.
Figure 8.
Total kilocalories of food consumed per day. MO = Menhaden Oil Diet, MF = Milk Fat Diet, OO = Olive Oil Diet. NS = No Significance, * p < 0.05
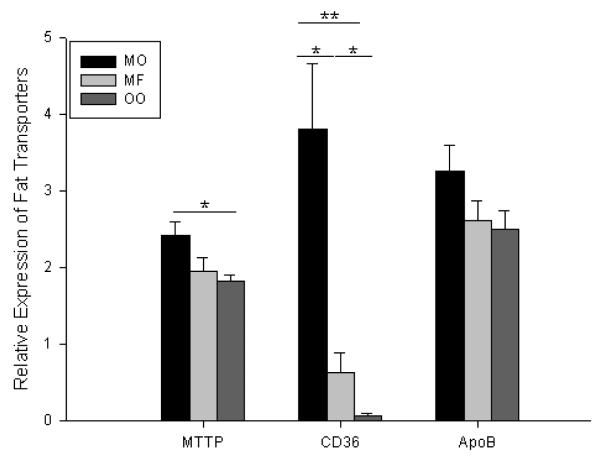
All mice demonstrated signs of adaptation with increased villus height after surgery with increases ranging from 54.0 to 82.7% compared to preoperative measurements (data not shown). There were no consistent statistically significant differences in villus height between groups. RT-PCR of ileal villus enterocytes for fat transporters revealed a modest increase in MTTP mRNA expression but no statistically significant difference in ApoB (Figure 9). However, there was a 50-fold and 6-fold increase in CD36 expression in the menhaden oil group when compared to that of olive oil and milk fat, respectively. Milk fat mice also experienced a 9-fold increase in CD36 expression compared to olive oil mice.
Figure 9.
Fat related mRNA expression of microsomal triglyceride transporter protein (MTTP), CD36, and apolipoprotein B (ApoB) as determined by RT-PCR. β–Actin was used as the endogenous control, and whole bowel was used as a calibrator. MO = Menhaden Oil Diet, MF = Milk Fat Diet, OO = Olive Oil Diet. * p < 0.05, ** p < 0.005
Discussion
We have found that an increased concentration of enteral fat (HFD) optimally prevented postoperative catabolic responses and increased lean mass after resection. In contrast, a high carbohydrate/low fat diet (LFD) resulted in the greatest proportion of body fat with the highest degree of carbohydrate transporter mRNA expression. Regardless of the diet, all mice experienced structural adaptation after SBR. Of the different types of fats, menhaden oil (polyunsaturated LCFA) produced the greatest percent lean mass with the least amount of weight loss after surgery as well as increased MTTP and CD36 expression.
High fat diets have been associated with increased structural adaptation after SBR,1-4 however, the amount of fat necessary to illicit this response had not previously been determined. Additionally, it was unknown if more enteral fat resulted in a greater response as in a dose-dependent pattern. We found that MFD led to significantly greater increases in villus height than HFD and LFD groups, who showed similar degrees of villus growth. This would suggest that although increased enteral fat of 44% kcal enhances adaptation, extreme levels of fat up to 71% kcal fat do not provide any further stimulation beyond adaptive growth. A previous study has found that rats given a low fat diet with 3% kcal/fat after SBR were found to have decreased body weight, mucosal weight, and attenuated villus height/proliferation/apoptosis in the ileum when compared to normal chow.5, 6 However, our results reveal that at least 10% kcal fat as in LFD is adequate to demonstrate adaptive changes.
Different compositions of high fat diets have been studied although often not in the same study. Menhaden oil, milk fat, and lard (a combination of saturated and monounsaturated LCFA) have all demonstrated augmented adaptation after SBR.1-4 Another study investigated two different kinds of polyunsaturated fatty acids, safflower oil or arachidonic acid/docosahexanoic acid (AA/DHA) (high in omega-3), and found that the AA/DHA diet had superior adaptive changes after SBR.3 When we investigated the effects of menhaden oil, milk fat, and olive oil simultaneous, all three kinds of fat were indistinguishable with regard to structural adaptation.
We also sought to investigate the metabolic changes that occur in response to resection. When different levels of fat were analyzed, we found that although the MFD group had the greatest increase in villus height, it did not have any greater increase in lean mass or weight gain compared to the other groups. Similarly, mice that consumed menhaden oil had the highest percent lean mass and greatest weight retention without having enhanced structural growth compared to the olive oil or milk fat groups. Thus, we found no correlation with between villus growth and retention of lean mass, as enhanced villus height after resection did not lead to increased percent lean mass. Similarly, there was also no association with increased villus height and increased postoperative weight gain. Thus, other factors affect metabolism besides villus height.
It is somewhat counterintuitive that mice consuming a HFD had the highest lean mass while mice that consumed a LFD had the greatest composition of body fat. Our HFD is similar to a ketogenic diet as it contains high fat with low carbohydrates. A previous study on nonoperated mice found that a ketogenic diet (79% fat, 0.76% carbohydrate) resulted in a decreased weight gain with reduced fat and lean mass compared to mice given a “high fat” diet (24% fat, 41% carbohydrates), despite consuming an equal amounts of calories.12 Having high levels of fat provides an alternative energy source (one that is also greater in energy density) than protein. Additionally, a ketogenic diet enables fat catabolism while sparing protein. It is possible that during the stress phase of surgery, fat may be used to drive carbohydrate production. In contrast, excess carbohydrates promote fatty acid synthesis, which may explain our results that mice with the greatest carbohydrate component (LFD) had the greatest weight gain as well as the greatest percent body fat. While it could be argued that perhaps enteral fat promotes protein absorption, the amount of protein in each diet was the same.
Menhaden oil is considered a good source of omega-3 fatty acids, eicosapentaenoic acid (EPA) and docosahexanoic acid (DHA). Omega-3 fatty acids are precursors for the synthesis of anti-inflammatory prostaglandins as well as important structural cellular component. There are multiple health benefits with omega-3 fatty acids, particularly with improved cardiovascular profiles. EPA and DHA have also been show to have protective effects on the intestine after ischemia-reperfusion injury.13 Omega-3 fatty acids have been found to be beneficial in terms of adaptation after SBR in animal models2, 3 and have been shown to decrease postoperative infections, wound complications, and length of stay in surgical patients.7-9 EPA has been shown to increase lean body mass in patients with head and neck cancer after surgery as well as in patients after esophageal cancer surgery.14, 15 It was theorized that antiinflammatory properties of EPA negate the proinflammatory cytokines that result in cachexia associated with cancer. Although our study did not measure cytokines, it is possible that the omega-3 fatty acids of menhaden oil may preserve lean body through the inhibition of proinflammatory cytokines.
The results from the examination of carbohydrate transporters were unsurprising as the mRNA expression of SGLT1 and GLUT2 significantly increased with the highest carbohydrate diet. SLGT1 is a sodium glucose cotransporter that mediates uptake of glucose across the brush border membrane while GLUT2 is a basolateral and apical transporter. Both SGLT1 and GLUT2 have been found to be upregulated with high carbohydrate diets and play an important role in carbohydrate absorption.16, 17 Additionally, SGLT1 is required for upregulation of GLUT2.18
Increased expression of fat transporters has been shown before in other studies of animals receiving a diet high in fat.19, 20 However, there have been no studies that have examined the expression of these intestinal lipid transporters at higher levels of fat or with different kinds of fat, especially in the context of SBR. We found that only the MFD had increased fat transporter mRNA expression in MTTP and CD36 while HFD had similar levels of expression to LFD. Additionally, menhaden oil had significantly greater expression of MTTP but especially CD36.
MTTP assists lipoprotein synthesis by shuttling the lipid to the ApoB protein. The increases in MTTP were mild and unlikely to be clinically significantly. However, CD36 had significant changes in expression, particularly with menhaden oil. CD36 is a facilitator of fatty acid uptake in the brush border membrane of the proximal intestine, but also in the heart, skeletal muscle, and adipose tissue.19, 21
It is possible to infer that CD36 may be involved in the preservation of lean mass due to the dramatic increase seen in the menhaden oil group. However, CD36 was not found to be dramatically elevated in mice fed HFD, who demonstrated the greatest lean mass. CD36-null mice do not have a net fat malabsorption and were protected against obesity when placed on a high fat diet. These mice were noted to have similar lean mass but decreased body fat.22 Muscle-specific CD36 transgenic mice were found to have leaner body compositions and less adipose tissue but also weighed less than wild type mice.23 If CD36 contributes to the beneficial metabolic effects of menhaden oil after resection, then we may expect to see an attenuation of this phenotype if this experiment were to be replicated with CD36-null mice.
This study elucidates the importance of enteral fat in maintaining lean body mass after surgery. While the mechanism is unclear, we hypothesize based on our findings that enteral omega-3 fatty acids reduce pro-inflammatory cytokines that may further promote catabolism. Optimizing fat-based enteral nutrition may be a useful adjunct in the treatment of short gut syndrome to maintain body weight and prevent protein loss.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 9th Annual Academic Surgical Congress in San Diego, CA, February 4-6, 2014
References
- 1.Sukhotnik I, Mor-Vaknin N, Drongowski RA, Miselevich I, Coran AG, Harmon CM. Effect of dietary fat on early morphological intestinal adaptation in a rat with short bowel syndrome. Pediatr Surg Int. 2004;20:419–424. doi: 10.1007/s00383-004-1168-9. [DOI] [PubMed] [Google Scholar]
- 2.Vanderhoof JA, Park JH, Herrington MK, Adrian TE. Effects of dietary menhaden oil on mucosal adaptation after small bowel resection in rats. Gastroenterology. 1994;106:94–99. doi: 10.1016/s0016-5085(94)94589-6. [DOI] [PubMed] [Google Scholar]
- 3.Kollman KA, Lien EL, Vanderhoof JA. Dietary lipids influence intestinal adaptation after massive bowel resection. J Pediatr Gastroenterol Nutr. 1999;28:41–45. doi: 10.1097/00005176-199901000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Choi PM, Sun RC, Guo J, Erwin CR, Warner BW. High-Fat Diet Enhances Villus Growth During the Adaptation Response to Massive Proximal Small Bowel Resection. J Gastrointest Surg. 2013 doi: 10.1007/s11605-013-2338-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sukhotnik I, Shiloni E, Krausz MM, Yakirevich E, Sabo E, Mogilner J, et al. Low-fat diet impairs postresection intestinal adaptation in a rat model of short bowel syndrome. J Pediatr Surg. 2003;38:1182–1187. doi: 10.1016/s0022-3468(03)00264-1. [DOI] [PubMed] [Google Scholar]
- 6.Sukhotnik I, Gork AS, Chen M, Drongowski RA, Coran AG, Harmon CM. Effect of low fat diet on lipid absorption and fatty-acid transport following bowel resection. Pediatr Surg Int. 2001;17:259–264. doi: 10.1007/s003830100590. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki D, Furukawa K, Kimura F, Shimizu H, Yoshidome H, Ohtsuka M, et al. Effects of perioperative immunonutrition on cell-mediated immunity, T helper type 1 (Th1)/Th2 differentiation, and Th17 response after pancreaticoduodenectomy. Surgery. 2010;148:573–581. doi: 10.1016/j.surg.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Marik PE, Zaloga GP. Immunonutrition in high-risk surgical patients: a systematic review and analysis of the literature. JPEN J Parenter Enteral Nutr. 2010;34:378–386. doi: 10.1177/0148607110362692. [DOI] [PubMed] [Google Scholar]
- 9.Pradelli L, Mayer K, Muscaritoli M, Heller AR. n-3 fatty acid-enriched parenteral nutrition regimens in elective surgical and ICU patients: a meta-analysis. Crit Care. 2012;16:R184. doi: 10.1186/cc11668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo J, Longshore S, Nair R, Warner BW. Retinoblastoma protein (pRb), but not p107 or p130, is required for maintenance of enterocyte quiescence and differentiation in small intestine. J Biol Chem. 2009;284:134–140. doi: 10.1074/jbc.M806133200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helmrath MA, VanderKolk WE, Can G, Erwin CR, Warner BW. Intestinal adaptation following massive small bowel resection in the mouse. J Am Coll Surg. 1996;183:441–449. [PubMed] [Google Scholar]
- 12.Kennedy AR, Pissios P, Otu H, Roberson R, Xue B, Asakura K, et al. A high-fat, ketogenic diet induces a unique metabolic state in mice. Am J Physiol Endocrinol Metab. 2007;292:E1724–1739. doi: 10.1152/ajpendo.00717.2006. [DOI] [PubMed] [Google Scholar]
- 13.Brahmbhatt V, Oliveira M, Briand M, Perrisseau G, Bastic Schmid V, Destaillats F, et al. Protective effects of dietary EPA and DHA on ischemia-reperfusion-induced intestinal stress. J Nutr Biochem. 2013;24:104–111. doi: 10.1016/j.jnutbio.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Weed HG, Ferguson ML, Gaff RL, Hustead DS, Nelson JL, Voss AC. Lean body mass gain in patients with head and neck squamous cell cancer treated perioperatively with a protein- and energy-dense nutritional supplement containing eicosapentaenoic acid. Head Neck. 2011;33:1027–1033. doi: 10.1002/hed.21580. [DOI] [PubMed] [Google Scholar]
- 15.Ryan AM, Reynolds JV, Healy L, Byrne M, Moore J, Brannelly N, et al. Enteral nutrition enriched with eicosapentaenoic acid (EPA) preserves lean body mass following esophageal cancer surgery: results of a double-blinded randomized controlled trial. Ann Surg. 2009;249:355–363. doi: 10.1097/SLA.0b013e31819a4789. [DOI] [PubMed] [Google Scholar]
- 16.Thomson AB, Keelan M, Thiesen A, Clandinin MT, Ropeleski M, Wild GE. Small bowel review: normal physiology part 1. Dig Dis Sci. 2001;46:2567–2587. doi: 10.1023/a:1012794505897. [DOI] [PubMed] [Google Scholar]
- 17.Kellett GL, Brot-Laroche E, Mace OJ, Leturque A. Sugar absorption in the intestine: the role of GLUT2. Annu Rev Nutr. 2008;28:35–54. doi: 10.1146/annurev.nutr.28.061807.155518. [DOI] [PubMed] [Google Scholar]
- 18.Gorboulev V, Schurmann A, Vallon V, Kipp H, Jaschke A, Klessen D, et al. Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes. 2012;61:187–196. doi: 10.2337/db11-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abumrad NA, Davidson NO. Role of the gut in lipid homeostasis. Physiol Rev. 2012;92:1061–1085. doi: 10.1152/physrev.00019.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynes M, Narisawa S, Millan JL, Widmaier EP. Interactions between CD36 and global intestinal alkaline phosphatase in mouse small intestine and effects of high-fat diet. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1738–1747. doi: 10.1152/ajpregu.00235.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nassir F, Wilson B, Han X, Gross RW, Abumrad NA. CD36 is important for fatty acid and cholesterol uptake by the proximal but not distal intestine. J Biol Chem. 2007;282:19493–19501. doi: 10.1074/jbc.M703330200. [DOI] [PubMed] [Google Scholar]
- 22.Hajri T, Hall AM, Jensen DR, Pietka TA, Drover VA, Tao H, et al. CD36-facilitated fatty acid uptake inhibits leptin production and signaling in adipose tissue. Diabetes. 2007;56:1872–1880. doi: 10.2337/db06-1699. [DOI] [PubMed] [Google Scholar]
- 23.Ibrahimi A, Bonen A, Blinn WD, Hajri T, Li X, Zhong K, et al. Muscle-specific overexpression of FAT/CD36 enhances fatty acid oxidation by contracting muscle, reduces plasma triglycerides and fatty acids, and increases plasma glucose and insulin. J Biol Chem. 1999;274:26761–26766. doi: 10.1074/jbc.274.38.26761. [DOI] [PubMed] [Google Scholar]