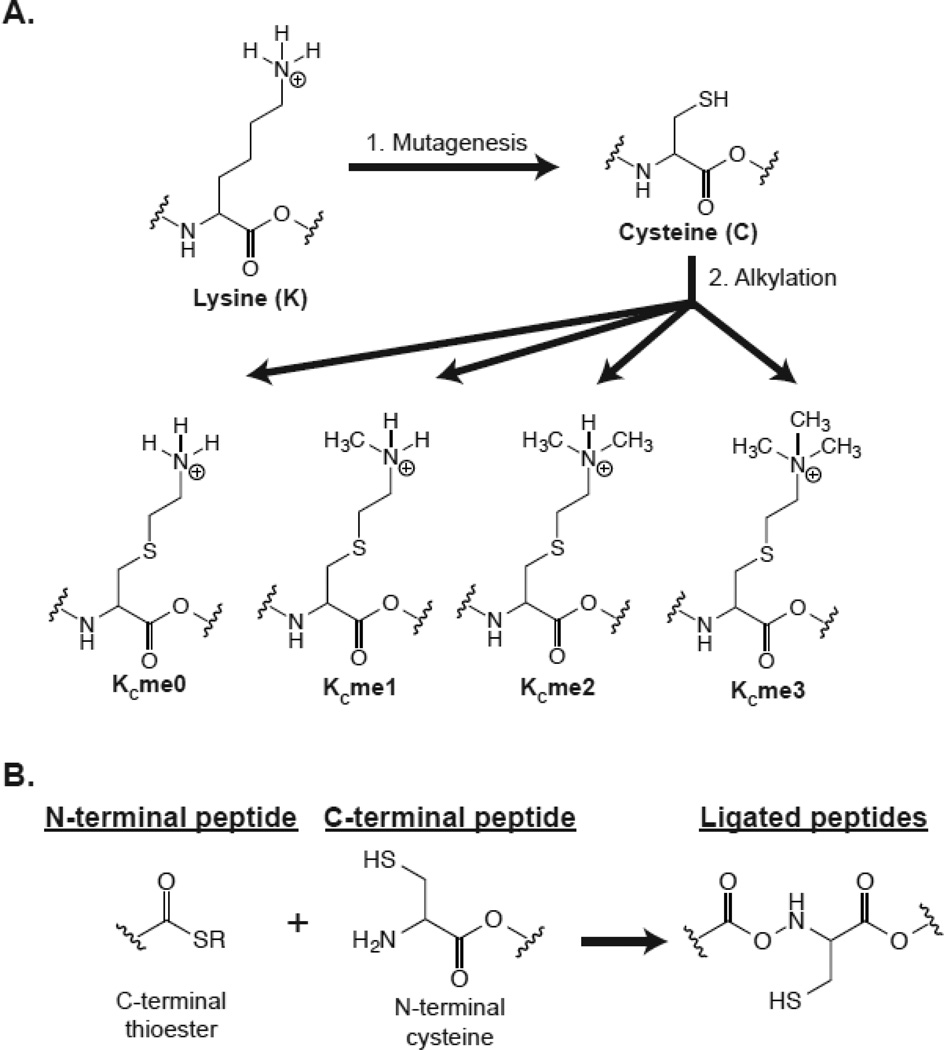
Figure 3.
Chemical strategies to generate specifically modified histone proteins. A) Modified lysine analog construction. All cysteines must be removed from a candidate protein, such as making an H3C110A mutation. The desired modified lysine is then mutated to cysteine and expressed in Escherichia coli. Following expression and purification, alkylating reagents are applied to the cysteines to generate a specific methyl lysine state at that particular residue. B) Protein ligation. Two peptides can be ligated if they contain the correct chemical signatures. The N-terminal peptide must contain a C-terminal thioester moiety that can either be generated by peptide synthesis or by using natural iteins. The C-terminal peptide must contain an N-terminal cysteine residue, which can be installed by peptide synthesis or molecular techniques such as protease cleavage. The chemoselective reaction will occur spontaneously to generate a ligated protein through a native peptide bond.