Figure 5. Protein Insertion into the Mitochondrial Outer Membrane.
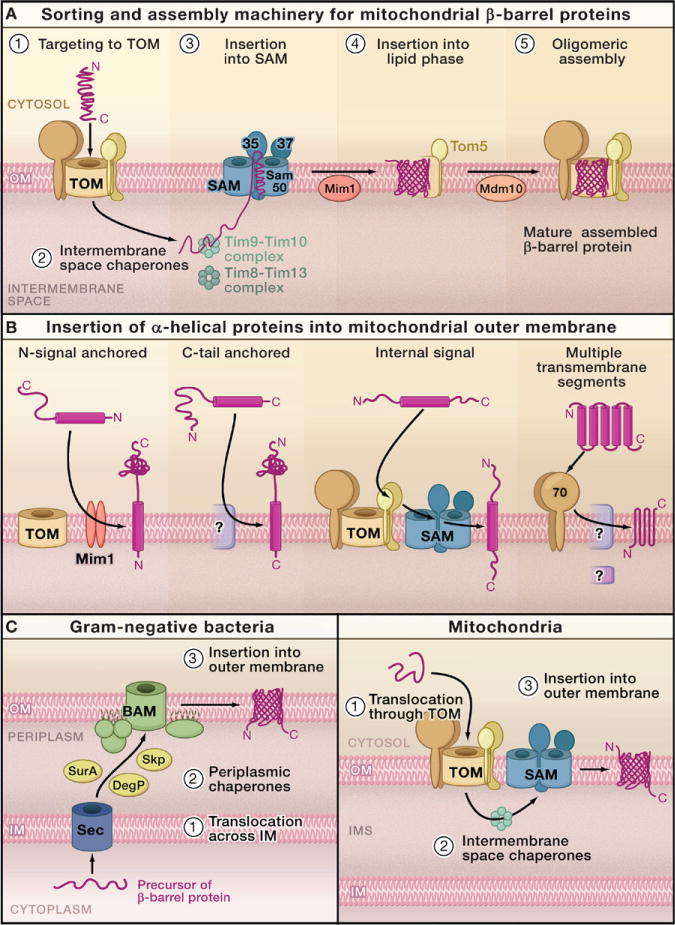
(A) Sorting of β-barrel proteins. The precursors of β-barrel proteins are initially transported via the translocase of the outer membrane (TOM complex) into the intermembrane space (IMS). IMS chaperone complexes transfer the precursors to the sorting and assembly machinery (SAM complex). The two essential subunits, Sam50 and Sam35, cooperate in insertion of the precursors into the outer membrane. Steps 1–3 show the general pathway for sorting of β-barrel proteins. Steps 4 and 5 depict specific additional steps of the assembly pathway of the β-barrel protein, Tom40. Mim1 supports integration of the Tom40 precursor into the outer membrane; Tom40 then associates with Tom5 and a further Tom40 molecule, followed by Mdm10-supported assembly of the oligomeric TOM complex.
(B) Insertion of α-helical proteins. Mitochondrial outer membrane proteins with α-helical transmembrane segments that typically function as sorting signals are imported by different pathways, depending on the location of the sorting signal. For different precursor proteins, a dependence on Mim1, Tom70, the TOM complex and/or SAM has been reported, but the mechanisms of membrane integration have not yet been defined.
(C) Evolutionary conservation of β-barrel sorting from Gram-negative bacteria to mitochondria. Bacteria synthesize the precursor proteins in the cytoplasm. The precursors are translocated through the Sec machinery to the periplasm and guided by chaperones to the β-barrel assembly machinery (BAM) of the outer membrane. For mitochondrial β-barrel proteins, the site of synthesis and the initial translocation through the TOM complex are different from bacteria, yet the subsequent steps of chaperone-guided transfer through the IMS and insertion into the outer membrane by the SAM complex share characteristics with the bacterial system. Sam50 is homologous to the central BAM component BamA (Omp85/YaeT). However, differences are also apparent as the other SAM and BAM subunits, as well as the chaperones of the IMS and periplasm, are not homologous to each other, and the lipid composition of the mitochondrial outer membrane is different from that of the bacterial outer membrane.
