Abstract
Thyroid hormone stimulates renal proximal tubule NaCl and NaHCO3 absorption in part by activating the apical membrane Na/H exchanger NHE3. We used a renal epithelial cell line, the opossum kidney (OK) cell, to define the mechanism by which 3,5,3′-triiodothyronine (T3) increases NHE3 activity. T3 stimulated NHE3 activity, an effect that was blocked by inhibition of cellular transcription or translation. The increase in activity was associated with increases in steady-state cell surface and total cellular NHE3 protein and NHE3 transcript abundance. T3 stimulated transcription of the NHE3 gene and had no effect on NHE3 transcript stability. The transcriptional activity of the 5′-flanking region of the rat NHE3 gene was stimulated by T3 when expressed in OK cells. When heterologously expressed rat NHE3 transcript levels were clamped constant with a constitutive promoter in OK cells, T3 has no effect on rat NHE3 protein abundance, suggesting the absence of regulation of NHE3 protein stability or translation. These studies demonstrate that T3 stimulates NHE3 activity by activating NHE3 gene transcription and increasing NHE3 transcript and protein abundance.
Keywords: proximal tubule, acid-base balance, NaCl homeostasis, development, promoter
Sodium Chloride and NaHCO3 absorption in the mammalian renal proximal tubule is affected by the thyroid status of the animal (10, 11, 21, 22). The developmental maturation of proximal tubule NaCl and NaHCO3 absorption is temporally preceded by an increase in circulating thyroid hormone levels (29). A significant portion of proximal tubule NaCl and NaHCO3 transport is mediated by apical membrane Na/H exchange (24, 25). Na/H exchange activity in renal cortical apical membrane vesicles is increased in hyperthyroid and decreased in hypothyroid animals (18, 19). Although the effect on apical membrane Na/H exchange may be mediated in part by changes in glomerular filtration rate, systemic hemodynamic and/or neurohumoral changes (17), a direct effect of thyroid hormone on the maximum velocity (Vmax) of the Na/H exchanger has been demonstrated in a cell culture model of the proximal tubule, the opossum kidney (OK) cell (30).
With the identification of Na/H exchanger isoform cDNAs (reviewed in Ref. 28), specific reagents are now available to address the mechanisms of regulation of proximal tubule Na/H exchange. The predominant isoform responsible for proximal tubule apical membrane Na/H exchange is NHE3 (1, 6), and an opossum NHE3 homologue is expressed in OK cells. Most chronic biological effects of thyroid hormone are believed to be mediated via activation of gene transcription (26). However, one study in rat kidney showed that changes in the NHE3 transcript in response to thyroid hormone are not accompanied by changes in NHE3 protein abundance, raising doubts as to the role of increased NHE3 transcript in mediating the increased NHE3 activity (2). In the present study, we found that thyroid hormone administration to adult rats increases both apical membrane Na/H exchanger activity and NHE3 protein abundance. We used OK cells that express NHE3 to further define the mechanisms by which thyroid hormone increases NHE3 activity. We showed that thyroid hormone increases NHE3 activity by activating the promoter and transcription of the NHE3 gene, leading to increased NHE3 transcript and protein abundance.
METHODS
Apical membrane preparation from hyperthyroid rats
Sprague-Dawley rats (150–200 g) with free access to food and water were given 100 μg/kg of 3,5,3′-triiodothyronine (T3; dissolved in 100 μl 0.1 mM NaOH and pH adjusted to 7.4 with HCl) intraperitoneally once daily for 4 days. Control animals were given an injection of the vehicle. Renal cortex was dissected and homogenized in buffer containing (in mM) 300 mannitol, 20 HEPES (pH 7.50), and 5 EGTA, as well as (in μg/ml) 100 phenylmethylsulfonyl fluoride (PMSF), 2 leupeptin, 2 aprotinin, and 2 pepstatin A, in a Brinkmann Polytron. The apical membrane vesicles were prepared from the cortical homogenate by exposing cortical membranes to three consecutive precipitations by 15 mM MgCl2, and the final apical membrane-enriched vesicles were pelleted from the supernatant (20,000 rpm, 40 min, 4°C; Beckman J2–21M, JA-20 rotor).
Cell culture
OK cells were maintained in DMEM supplemented with 4.5 mg/ml glucose as well as 100 U/ml penicillin and 100 μg/ml streptomycin (penicillin-streptomycin). Confluent monolayers on glass coverslips (NHE activity) or plastic petri dishes (all other studies) were rendered quiescent by serum removal for 48 h before study. T3 was dissolved in 10−2 M NaOH that was diluted 105-fold in culture medium during experiments (final concentration = 10−7 M). Control cells received similar amounts of NaOH as T3-treated cells. For studies examining constitutively transcribed heterologous rat NHE3, rat NHE3 was cloned in pcDNA-1 (Invitrogen, San Diego, CA) and transfected into OK cells by CaPO4 precipitation along with pSV40Neo to confer neomycin resistance, and the transfectants were selected (400 μg/ml) and maintained (200 μg/ml) in G418. G418 was replaced with penicillin-streptomycin two passages before studies.
NHE3 activity, immunoreactive protein, and transcripts
NHE3 activity in OK cells was measured fluorometrically by the pH-sensitive dye 2′,7′-bis(2-carboxyethyl)-5(6)-carboxy-fluorescein (10 μM) in the absence of HCO3/CO2 as Na-dependent (Na concn = 138 mM) cell pH (λex 500/450 and λem 530 nm) recovery (dpHi/dt; pH units/min) after acid loading with the K/H ionophore nigericin. For measurement of Na/H exchange activity in rat renal apical membranes, 200 μg of vesicles were acid loaded (in mM: 300 mannitol, 20 MES, pH 5.5) and exposed to 10× volume uptake solution [in mM: 300 mannitol, 20 Tris (pH 7.5), 0.1 22NaCl] to initiate transport at 20°C for 10 s. Uptake was stopped by dilution with stop solution (in mM: 150 NaCl, 20 Tris, pH 7.5) at 4°C, and 22Na uptake was quantified by rapid filtration on 0.65 μM Milli-pore filters and by scintillation counting.
The NHE3 antigen was quantified by immunoblots. Total cellular NHE3 was measured by lysing cells in membrane buffer [150 mM NaCl, 50 mM Tris (pH 7.5), 5 mM EDTA, 100 μg/ml PMSF, 4 μg/ml aprotinin, and 4 μg/ml leupeptin], and the membrane fraction was pelleted (109,000 g at rmax 50,000 rpm for 25 min on a Beckman TLX:TLA 100.3 rotor at 4°C). Twenty micrograms of either OK cell or rat renal cortical apical membrane were fractionated by SDS-PAGE and transferred to nitrocellulose filters. To measure cell surface NHE3, surface proteins were labeled with biotin [10 mM triethanolamine (pH 7.4), 2 mM CaCl2, 150 mM NaCl, and 1.5 mg/ml sulfosuccinimidyl 2-(biotinamido)ethyl-1,3-dithiopropionate; Pierce, Rockford, IL] for 30 min at 4°C. After quenching [in mM: 140 Na2HPO4 (pH 7.4), 0.1 CaCl2, 1 MgCl2, 100 glycine], cells were lysed in RIPA buffer [150 mM NaCl, 50 mM Tris·HCl (pH 7.4), 0.5 mM EDTA, 80 mM NaF, 25 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 1% Triton X-100 (vol/vol), 0.5% deoxycholate (wt/vol), 0.1% SDS (wt/vol), 250 μg/ml PMSF, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 20 μg/ml pepstatin], and an aliquot of the whole cell lysate was saved for simultaneous measurement of changes in whole cell NHE3 in the same cells. The remainder of the lysate was equilibrated with streptavidin-agarose at 4°C on a rotary mixer overnight. After being washed with RIPA, biotinylated surface proteins were released from the biotinstreptavidin/agarose complex by 100 mM dithiothreitol, and the liberated proteins were retrieved as a supernatant after centrifugation. Anti-OK NHE3 antiserum (no. 5683, raised against a fusion protein of maltose binding protein and OK NHE3 amino acids 484–839) was used at 1:500 dilution. The specificity of the antisera was confirmed by complete blockade of labeling by the immunogenic fusion protein but not maltose binding protein (data not shown). Several anti-rat NHE3 antisera were used: no. 1568 (against epitope DSFLQADGPEEQLQ at 1:1,000 dilution), no. 1566 (against epitope YSRHELTPNEDEKQ at 1:1,000 dilution), and no. 1313 (against a fusion protein of maltose binding protein and rat NHE3 amino acids 405–831 at 1:400 dilution). After incubation with horseradish peroxidase-coupled mouse anti-rabbit secondary antibody, signals were detected by enhanced chemiluminescence (Amersham, Arlington Heights, IL) and quantified by densitometry.
NHE3 transcript abundance was measured by RNA blots. Monolayers were lysed in GTC solution [4 M guanidinium thiocyanate, 0.1 M 2-mercaptoethanol, 0.025 M sodium citrate (pH 7.0), 0.5% (wt/vol) N-lauroylsarcosine], and RNA was extracted with phenol-chloroform, precipitated with ethanol, size fractionated with formaldehyde gel electrophoresis, transferred to a nylon membrane, and hybridized with a 32P-labeled full-length coding OK NHE3 cDNA (0.3-kb 5′UTR + 2.5-kb coding) or a 1.2-kb Pst I fragment of the rat NHE3 cDNA. Hybridization conditions were exactly as previously described (4).
NHE3 transcript half-life and transcription rate
To determine the effect of T3 on the half-life of NHE3 mRNA, OK cells were treated with T3 or vehicle for 16 h, and then 10−5 M actinomycin was added to completely inhibit transcription. Preliminary studies have documented that this dose of actinomycin inhibits transcription in OK cells by 98% (data not shown). Cells were harvested at the designated time points for NHE3 transcript quantification by RNA blots. The in vitro transcription rate was measured by nuclear run-on assay as previously described (4). Briefly, after treatment with T3 or vehicle for 4 h, cells were scraped, washed in PBS at 4°C, and lysed [10 mM NaCl, 2 mM MgCl2, 10 mM Tris·HCl (pH 7.4), and 0.5% (vol/vol) Nonidet P-40]. Nuclei were pelleted (500 g for 5 min at 45°C), washed, resuspended in reaction buffer (140 mM KCl, 20 mM Tris·HCl, pH 7.5, 10 mM MgCl2, 13.4 mM 2-mercaptoethanol, 1 mM MgCl2, 0.9 mM of each NTP, 10 mM phosphocreatine, 10 μg phosphocreatine kinase, and 250 μCi [32P]UTP), and in vitro transcription was allowed to proceed at 30°C for 30 min. Nuclei were then lysed by shearing in high-salt buffer (in mM: 500 NaCl, 2 CaCl2, 50 MgCl2, 10 Tris·HCl, pH 7.5) and treated with RNase-free DNase (20 units at 30°C for 30 min), and the nuclear nascent RNA was recovered by phenol-chloroform extraction and LiCl precipitation and was resuspended in NETS buffer (in mM: 200 NaCl, 10 EDTA, 10 Tris·HCl, pH 7.4). After brief NaOH hydrolysis, the radiolabeled RNA was hybridized for 48 h to linearized full-length coding OK NHE3 cDNA, along with pBluescript and β-actin controls immobilized on nylon filters by slot blotting (20 μg DNA/slot). Hybridization and washing conditions were exactly as previously described (4).
Activity of NHE3 5′-flanking region
Promoter-reporter constructs containing 2.2 or 0.82 kb of the 5′-flanking region of the rat NHE3 gene cloned upstream to the chloramphenicol acetyltransferase (CAT) gene in the vector pBLCAT3 were used for these studies. Transfections were performed by CaPO4 precipitation along with a pTK-gal plasmid to normalize for transfection efficiency. Forty hours posttransfection, cells were treated with 10−7 M T3 or vehicle for 4 h, washed in PBS, and lysed by shearing (250 mM Tris·HCl, pH 7.8). Lysate supernatant (5,000 g for 10 min at 4°C) expressing equivalent amounts of β-galactosidase activity was incubated in CAT reaction buffer (500 mM Tris·HCl, pH 7.8, 0.53 mM acetyl-CoA, 0.1 μCi [14C]chloramphenicol), and chloramphenicol and its acetylated products were extracted by ethyl acetate, resolved by TLC [19:1 (vol/vol) chloroform-methanol], and identified by autoradiography.
RESULTS
T3 increases apical membrane Na/H activity and NHE3 protein abundance in rat cortex
Four days of T3 administration increased apical membrane NHE activity by 45% (means ± SE in pmol·mg protein−1·10 s−1: euthyroid, 521 ± 27, n = 3; hyperthyroid, 728 ± 45, n = 3; P < 0.05, t-test). NHE3 antigen was quantified in apical membranes from euthyroid and hyperthyroid animals with three independent antisera, and the result using antiserum no. 1568 is shown in Fig. 1. Apical membrane NHE3 antigen was increased by 83% (P < 0.05, t-test; control, n = 3; T3, n = 4) in hyperthyroid animals. Antisera nos. 1566 and 1313 revealed similar results (data not shown). We next examined the mechanism of this stimulation in more detail in a cell culture model.
Fig. 1.
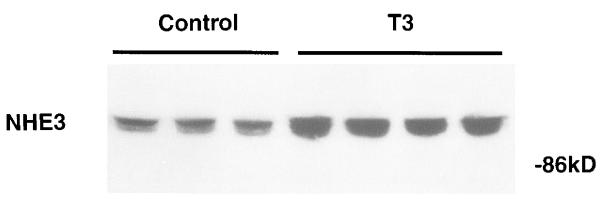
Effect of 3,5,3′-triiodothyronine (T3) on NHE3 protein abundance in rat cortical apical membranes. Rats rendered hyperthyroid with 100 μg·kg−1·day−1 T3 for 4 days were compared with euthyroid controls given vehicle (Con). NHE3 antigen in 20 μg renal cortical apical membrane protein was quantified by immunoblot using antiserum no. 1568. Mobility (in kDa) is shown at right.
Stimulation of NHE3 activity by T3 is dependent on intact RNA and protein synthesis in OK cells
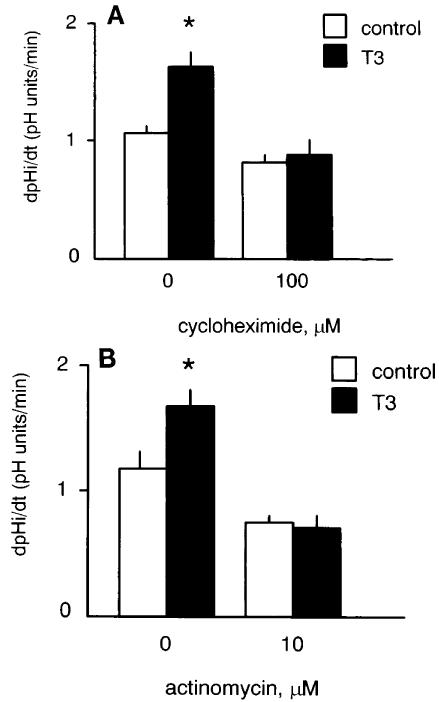
As shown previously by Yonemura and co-workers (30), addition of T3 directly stimulated NHE3 activity in OK cells (Fig. 2, A and B). In the presence of either actinomycin or cycloheximide, T3 had no effect on NHE3 activity, suggesting that the stimulation is dependent on intact transcription and translation. However, sensitivity to actinomycin and cycloheximide does not necessarily indicate that transcription and translation of NHE3 mediate the stimulation of NHE3 activity by T3.
Fig. 2.
Effect of T3 on NHE3 activity in opossum kidney (OK) cells: dependence on translation and transcription. Confluent quiescent OK cells were treated with 10−7 M T3 or vehicle for 24 h, and NHE3 activity was measured fluorometrically as Na-dependent cell pH recovery after an acid load (dpHi/dt). The T3 effect was studied in the absence or presence of 100 μM cycloheximide (A) or 10 μM actinomycin (B). *P < 0.05 (control vs. T3, unpaired t-test).
T3 increases NHE3 mRNA and immunoreactive protein abundance
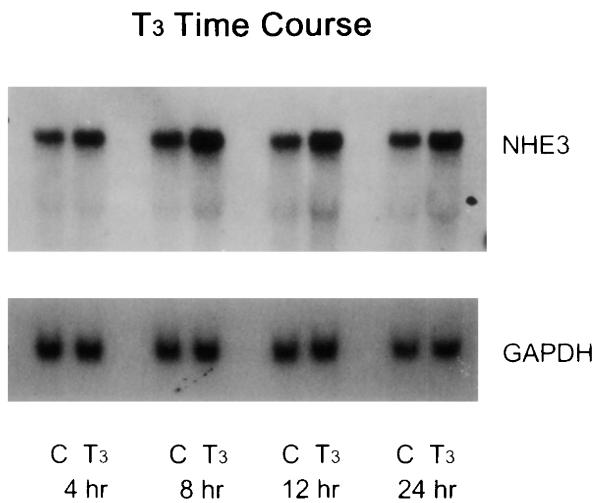
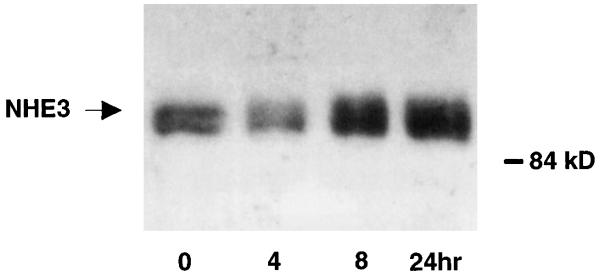
In both brush-border vesicles and OK cells, T3 activates Na/H exchange by increasing its Vmax (18, 30), a kinetic effect that is compatible with increased transporter protein abundance. To further define the mechanisms of the increase, we examined steady-state NHE3 mRNA and protein levels in response to T3 in OK cells. A typical experiment of the time course of the T3 effect on NHE3 mRNA is shown in Fig. 3. An increase in NHE3 mRNA was detectable as early as 4 h and persisted to 24 h (% increases, means ± SE, n = 4 for each time point: 4 h, 80 ± 15%, P < 0.05; 8 h, 125 ± 31%, P < 0.05; 12 h, 180 ± 25%; 24 h, 175 ± 75%, P < 0.05). As shown in Fig. 4, increased NHE3 protein was evident at 8 h and further increased at 24 h (% increases, mean ± SE, n = 4 for each time point: 4 h, 5 ± 15%, not significant; 8 h, 110 ± 35%, P < 0.05; 24 h, 230 ± 86%, P < 0.05). Next, the effect of T3 on cell surface and total cellular NHE3 was simultaneously measured in the same cells. In this set of experiments, T3 increased surface NHE3 by 110 ± 14% (n = 4, P < 0.05) and total cellular NHE3 by 207 ± 43% (n = 4, P < 0.05) in the same cells (Fig. 5).
Fig. 3.
Effect of T3 on NHE3 mRNA levels in OK cells. Confluent quiescent OK cells were treated with 10−7 M T3 or vehicle (C) for the designated period of time, and NHE3 transcript abundance in 20 μg total RNA was quantified by RNA blot. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as loading control. Four independent experiments showed similar results. NHE3 transcript, 9.5 kb; GAPDH transcript, 1.9 kb.
Fig. 4.
Effect of T3 on NHE3 antigen abundance in OK cells. Confluent quiescent OK cells were treated with 10−7 M T3 for stated period of time, and NHE3 antigen abundance in 20 μg of cell membranes was quantified by immunoblot. Mobility (in kDa) is shown on right. Four independent experiments showed similar results.
Fig. 5.
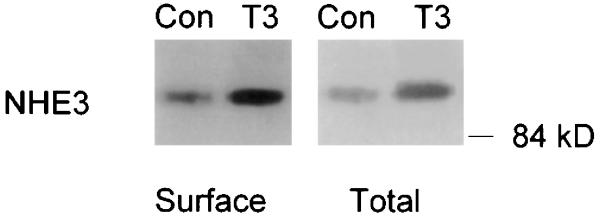
Effect of T3 on surface and total cellular NHE3 antigen in OK cells. Confluent quiescent OK cells were treated with 10−7 M T3 for 24 h, and surface and total cellular proteins were retrieved separately as the biotinylated protein fraction and total cell lysate, respectively. NHE3 antigen abundance was quantified by immunoblot. Mobility (in kDa) is shown on right. Four independent experiments showed similar results.
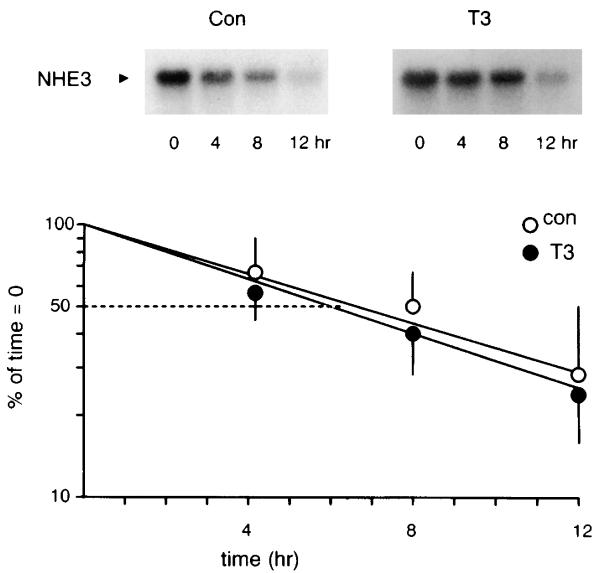
T3 stimulates NHE3 transcription without affecting transcript half-life
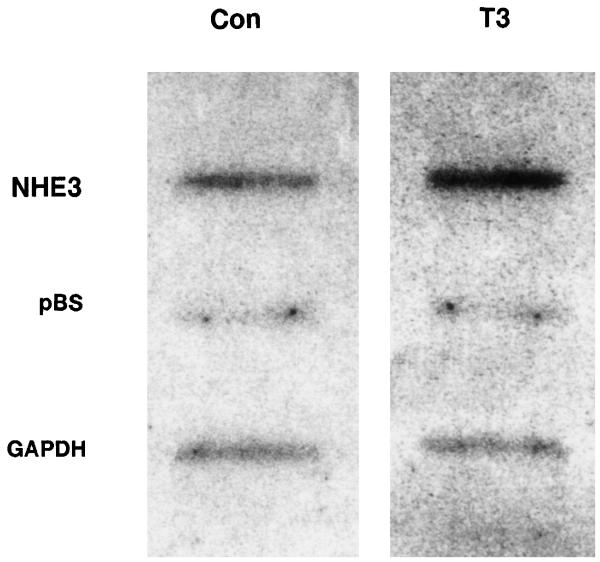
Increased steady-state NHE3 mRNA can be due to increased gene transcription, stabilization of transcripts, or both. In certain thyroid hormone-responsive genes, message stability has been proposed as a mechanism of regulation of increased transcript abundance (9, 15, 23, 27). We next addressed these two possibilities by directly measuring the effect of T3 on NHE3 transcript half-life and transcription rate. Figure 6 shows that, although T3 increased NHE3 transcript abundance, it had no effect on the rate of decay of NHE3 mRNA when transcription was completely inhibited. This suggests that the increase in NHE3 mRNA abundance is likely due to an increase in transcription. An in vitro transcription assay confirmed that T3 treatment increased the NHE3 transcription rate by about twofold (207 ± 35%, n = 3, P < 0.05; Fig. 7).
Fig. 6.
Effect of T3 on NHE3 mRNA half-life. OK cells were treated with 10−7 M T3 or vehicle for 4 h, and NHE3 mRNA decay was determined by RNA blots at indicated times in the presence of 10 μM actinomycin to inhibit transcription. Graph quantifies time-dependent decay of NHE3 transcripts expressed as percentage of level before addition of actinomycin. Circles and error bars depict means ± SE from 3 independent experiments.
Fig. 7.
Effect of T3 on NHE3 transcription rate. Nuclei were isolated from OK cells treated with T3 or vehicle for 4 h, and in vitro transcription rates were determined by nuclear run-on assay. Transcription rates for NHE3 and GAPDH are shown along with the background control with plasmid pBluescript (pBS). Three independent experiments showed similar results.
T3 trans-activates the 5′-flanking region of the NHE3 gene
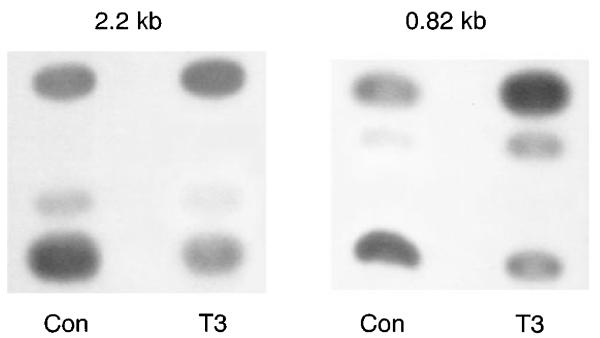
Most, although not all, transcriptional activation of genes is mediated by regulatory sequences 5′ to the coding region. We have isolated fragments of the rat NHE3 5′-flanking region that are transcriptionally competent in cis with reporter genes in OK cells (8). We next tested the effect of T3 on two fragments of the 5′-flanking region of the rat NHE3 gene in OK cells. Figure 8 shows that T3 activates both of these promoter fragments in OK cells.
Fig. 8.
Effect of T3 on 5′-flanking region of the NHE3 gene. Two chloramphenicol acetyltransferase (CAT) reporter constructs containing either 2.2 or 0.82 kb of the 5′-flanking region of the rat NHE3 gene were transiently transfected into OK cells along with a β-galactosidase-expressing plasmid. OK cells were treated with 10−7 M T3 or vehicle for 4 h, and CAT activity was assayed in cell lysates with equivalent amounts of β-galactosidase activity. Four (for 0.82 kb) and 2 (for 2.2 kb) independent experiments showed similar results.
T3 does not affect NHE3 protein half-life or translation
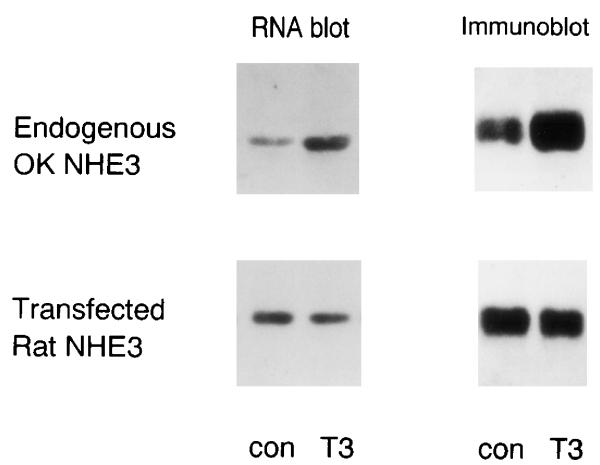
The data thus far unequivocally establish that T3 activates NHE3 gene transcription. The possibility remains that T3 may have an additional effect on NHE3 protein translation or stability. In the presence of an increased pool of translatable NHE3 mRNA, it is difficult to ascertain whether T3 directly activates translation of NHE3. To address this possibility, we expressed rat NHE3 in OK cells driven by a constitutive cytomegalovirus promoter that clamps the transcript of the transfected gene constant. If T3 increases NHE3 protein stability or translation, protein abundance of the transfected rat NHE3 should be increased by T3. Figure 8 shows that, in the absence of increased rat NHE3 mRNA, T3 has no effect on rat NHE3 protein abundance. The native opossum NHE3 message and protein serve as positive controls (Fig. 9). This suggests that the T3-induced increase in NHE3 protein abundance is mediated solely by increased NHE3 transcription.
Fig. 9.
Effect of T3 on native and transfected NHE3 protein and transcript abundance. Rat NHE3 was expressed in OK cells driven by a constitutive cytomegalovirus promoter to secure a constant level of transcripts. T3 was added for 24 h, and RNA and immunoblot analyses were performed for native opossum and heterologous rat NHE3 with species-specific cDNA probes and anti-NHE3 antisera. Four independent experiments showed similar results.
DISCUSSION
Thyroid hormone exerts a multitude of effects on extrarenal systems that can secondarily influence renal function (17). Even within the kidney, alterations in structural and absorptive capacity in the proximal tubule in hypothyroid and hyperthyroid animals may be partially accounted for by the reduction and augmentation of the glomerular filtration rate, respectively (10, 11, 17, 21, 22). The first unequivocal demonstration of a direct tubular effect was shown by Yonemura and co-workers (30), using the OK cell culture model. The first study that attempted to address the molecular mechanism of increased apical membrane Na/H exchange was in adult rats (2). In this study, although NHE3 mRNA abundance was significantly higher in hyperthyroid compared with euthyroid and hypothyroid rats, there was no difference in renal cortical membrane NHE3 protein abundance in hypothyroid, euthyroid, and hyperthyroid rats. This finding casts doubts on the role of increased NHE3 gene expression in mediating increased Na/H exchange activity. Indeed, one study had suggested that the apparent increase in Na/H exchange in apical membrane vesicles in hyper-thyroid animals can be accounted for by increased parallel Na and H conductances (20).
In the current study, we found that T3 increased both NHE3 activity and protein in both rat renal cortex and OK cells. In OK cells, the time profile of the T3-induced increase in NHE3 protein in this study is almost identical to that of increased Na/H exchange activity as defined by Yonemura and co-workers (30). However, the increase in total NHE3 protein abundance was much larger than the increase in NHE3 activity. There are two possible explanations. First, the T3-induced increase in NHE3 protein is much larger in the intracellular than the plasma membrane pool. Second, the increase in NHE3 protein is likely not linearly reflected by the dpHi/dt functional assay. The increased NHE3 protein is preceded by increased NHE3 transcript. Significant increases in NHE3 transcript can be detected as early as 4 h after T3 treatment. We demonstrate that the increase in NHE3 transcript is not due to increased transcript stability but rather to increased transcription of the NHE3 gene. This is in contrast to other thyroid-responsive genes such as the Na-K-ATPase α-and β-subunits and malic enzyme, where large increments in transcript levels were associated with little or no changes in transcription rate (3, 9, 12, 15). However, the main discrepancy between our study and that of Azuma and co-workers (2) is that we found an increase in NHE3 protein in adult rat cortex caused by T3 using three independent antisera. In a recent study, we showed in neonatal rats that hyperthyroidism is associated with increased, whereas hypothyroidism is associated with decreased, NHE3 transcript, protein, and activity in renal cortex (5). These findings in whole animals strongly support the physiological relevance of the current cell culture model.
To investigate the activation of NHE3 gene transcription, we examined NHE3 genomic sequences with a CAT reporter in OK cells. The 5′-flanking sequence of the rat NHE3 gene has been identified and shown to be active in the cis configuration in reporter constructs in renal epithelial cells (8, 16). In addition, trans-activation of the 5′-flanking sequences by glucocorticoids has been demonstrated (8, 16). In the present study, we show that both the 2.2- and 0.82-kb promoter fragments are activated by T3. Thus far we have not detected differences of sufficient magnitude and consistency between the 2.2- and 0.82-kb constructs to draw conclusions on relative T3 sensitivity. We previously demonstrated low baseline expression of both of these constructs in fibroblasts. Addition of T3 to 3T3 fibroblasts transiently transfected with the 2.2-kb promoter construct, along with a plasmid expressing the thyroid hormone receptor-α (THR-α; gift from Dr. Kevin Petty, University of Southwestern Medical Center, Dallas, TX) failed to elicit measurable CAT activity (data not shown), indicating that T3/THR-α per se is insufficient to activate the NHE3 promoter in a nonepithelial cellular context. The 2.2-kb nucleotide sequence upstream to the transcriptional start site harbors multiple consensus hexamers that conform partially to classical thyroid-responsive elements (TREs) (7, 8, 14, 16, 26). Relative to glucocorticoid-responsive elements (GREs), TREs in general tolerate a greater flexibility in half-site primary sequence variability and orientation (7). However, two half-sites in tandem are usually required for cis-activation (7, 13). The closest TRE half-sites in tandem in the NHE3 promoter are separated by 10 nucleotides. The ability of the 0.82-kb fragment that contains only four TRE half-sites to respond to T3 suggests that the T3 response may involve multiple transcription factors other than the THRs and their cognate dimerization partners.
In addition to gene transcription and message stability, it has been proposed, although not proven, that T3 can increase specific protein expression via either stabilization of protein or direct stimulation of translation (9, 27). Even if T3 directly activates NHE3 translation, it is extremely difficult to interpret an increase in NHE3 translation rate in the presence of an expanded pool of translatable NHE3 transcripts. We elected to create a situation in vivo where mRNA was clamped constant by expressing the rat NHE3 gene using a constitutive promoter. When rat NHE3 mRNA levels were kept constant, T3 had no effect on rat NHE3 abundance, even though the cells preserve an intact response to T3 as manifested by the rise in native OK NHE3 transcript and protein levels. This suggests that T3 exerts a minimal effect on NHE3 protein translation and stability. We recognize that the present data do not exclude the alternative explanation that the required regulatory sequences for translational regulation may reside in untranslated regions that are missing in the rat NHE3 cDNA construct.
In summary, we demonstrate that T3 increases proximal tubule apical membrane Na/H exchange by directly activating NHE3 gene transcription, and at least part of the regulatory sequence for this activation resides in the 5′-flanking region of the NHE3 gene. This may be an important mechanism in the determination of proximal tubule NaCl and NaHCO3 absorption in hyperthyroid and hypothyroid states and during the maturation of the proximal nephron.
Acknowledgments
We acknowledge the technical assistance of Sherry Scobee, Daniel Harris, Vangipuram Dwarakanath, and Ladonna Crowder. We thank Dr. David Russell and Dr. Robert Alpern for their helpful discussions and Dr. Alpern for his careful reading of the manuscript.
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-41612 (M. Baum) and DK-48482 (O. W. Moe) and by the Department of Veterans Affairs Research Service (O. W. Moe). A. Cano was a recipient of an American Heart Association Clinician Scientist Award.
REFERENCES
- 1.Amemiya M, Loffing J, Lötscher M, Kaissling B, Alpern RJ, Moe OW. Expression of NHE-3 in the apical membrane of rat renal proximal tubule and thick ascending limb. Kidney Int. 1995;48:1206–1215. doi: 10.1038/ki.1995.404. [DOI] [PubMed] [Google Scholar]
- 2.Azuma KK, Balkovetz DF, Magyar DE, Lescale-Matys L, Zhang Y, Chambrey R, Warnock DG, McDonough AA. Renal Na+/H+ exchanger isoforms and their regulation by thyroid hormone. Am. J. Physiol. 1996;270(39):C585–C592. doi: 10.1152/ajpcell.1996.270.2.C585. Cell Physiol. [DOI] [PubMed] [Google Scholar]
- 3.Back DW, Wilson SB, Morris SM, Jr., Goodridge AG. Hormonal regulation of lipogenic enzymes in chick embryo hepatocytes in culture. Thyroid hormone and glucagon regulate malic enzyme mRNA level at post-transcriptional steps. J. Biol. Chem. 1986;261:12555–12561. [PubMed] [Google Scholar]
- 4.Baum M, Amemiya M, Dwarakanath V, Alpern RJ, Moe OW. Glucocorticoids regulate NHE-3 transcription in OKP cells. Am. J. Physiol. 1996;270(39):F164–F169. doi: 10.1152/ajprenal.1996.270.1.F164. Renal Fluid Electrolyte Physiol. [DOI] [PubMed] [Google Scholar]
- 5.Baum M, Dwarakanath V, Alpern RJ, Moe OW. Effects of thyroid hormone on neonatal renal cortical Na/H antiporter. Kidney Int. 1998;53:1254–1258. doi: 10.1046/j.1523-1755.1998.00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biemesderfer D, Pizzonia J, Abu-Alfa A, Exner M, Reilly R, Igarashi P, Aronson PS. NHE3: a Na+/H+ exchanger isoform of renal brush border. Am. J. Physiol. 1993;265(34):F736–F742. doi: 10.1152/ajprenal.1993.265.5.F736. Renal Fluid Electrolyte Physiol. [DOI] [PubMed] [Google Scholar]
- 7.Brent GA, Harney JW, Chen Y, Warne RL, Moore DD, Larsen PR. Mutations of the rat growth hormone promoter which increase and decrease response to thyroid hormone define a consensus thyroid hormone response element. Mol. Endocrinol. 1989;3:1996–2004. doi: 10.1210/mend-3-12-1996. [DOI] [PubMed] [Google Scholar]
- 8.Cano A. Characterization of the rat NHE-3 promoter. Am. J. Physiol. 1996;271(40):F629–F636. doi: 10.1152/ajprenal.1996.271.3.F629. Renal Fluid Electrolyte Physiol. [DOI] [PubMed] [Google Scholar]
- 9.Couthésy-Theulaz I, Mérillat A-M, Honegger P, Rossier BC. Differential regulation of Na-K-ATPase isoform gene expression by T3 during rat brain developement. Am. J. Physiol. 1991;261(30):C124–C131. doi: 10.1152/ajpcell.1991.261.1.C124. Cell Physiol. [DOI] [PubMed] [Google Scholar]
- 10.De Santo NG, Capasso G, Kinne R, Moewes B, Carella C, Anastasio P, Giordano C. Tubular transport processes in proximal tubules of hypothyroid rats: lack of relationship between thyroidal dependent rise of isotonic fluid reabsorption and Na+-K+-ATPase activity. Pflügers Arch. 1982;394:294–301. doi: 10.1007/BF00583693. [DOI] [PubMed] [Google Scholar]
- 11.De Santo NG, Capasso G, Paduano C, Carella C, Giordano C. Tubular transport processes in proximal tubules of hypothyroid rats. Pflügers Arch. 1980;384:117–122. doi: 10.1007/BF00584426. [DOI] [PubMed] [Google Scholar]
- 12.Dosin B, Magnuson MA, Nikodem VM. Thyroid hormone regulation of malic enzyme synthesis. J. Biol. Chem. 1986;261:2250, 2257. [PubMed] [Google Scholar]
- 13.Feng J, Orlowski J, Lingrel JB. Identification of a functional thyroid hormone response element in the upstream flanking region of the human Na-K-ATPase 1 gene. Nucleic Acids Res. 1993;21:2619–2626. doi: 10.1093/nar/21.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flink IL, Morkin E. Interaction of thyroid hormone receptor with strong and weak cis-acting elements in the human α-myosin heavy chain gene promoter. J. Biol. Chem. 1990;265:11233–11237. [PubMed] [Google Scholar]
- 15.Gick GG, Ismail-Beigi F, Edelman IS. Thyroidal regulation of rat renal and hepatic Na-K-ATPase gene expression. J. Biol. Chem. 1988;163:16610–16618. [PubMed] [Google Scholar]
- 16.Kandasamy RA, Orlowski J. Genomic organization and glucocorticoid transcriptional activation of the Na/H exchanger NHE-3 gene. J. Biol. Chem. 1996;271:10551–10559. doi: 10.1074/jbc.271.18.10551. [DOI] [PubMed] [Google Scholar]
- 17.Katz AE, Emmanouel DS, Lindheimer MD. Thyroid hormone and the kidney. Nephron. 1975;15:223–249. doi: 10.1159/000180514. [DOI] [PubMed] [Google Scholar]
- 18.Kinsella J, Cujdik T, Sacktor B. Kinetic studies on the stimulation of Na/H exchange activity in renal brush border membranes isolated from thyroid hormone-treated rats. J. Membr. Biol. 1986;91:183–191. doi: 10.1007/BF01925795. [DOI] [PubMed] [Google Scholar]
- 19.Kinsella J, Sacktor B. Thyroid hormones increase Na+-H+ exchange activity in renal brush border membranes. Proc. Natl. Acad. Sci. USA. 1985;82:3606–3610. doi: 10.1073/pnas.82.11.3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macković-Basić M, Salihagić A, Ries N, Sabolić I. Absence of increased electroneutral Na+/H+ exchange in renal cortical brush-border membranes from hyperthyroid rats. Biochem. Pharmacol. 1988;37:1699–1705. doi: 10.1016/0006-2952(88)90431-5. [DOI] [PubMed] [Google Scholar]
- 21.Michael UF, Barenberg RL, Chavez R, Vaamonde CA, Papper S. Renal handling of sodium and water in hypothyroid rat. Clearance and micropuncture studies. J. Clin. Invest. 1972;51:1405–1412. doi: 10.1172/JCI106936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michael UF, Kelly J, Vaamonde CA. Impaired renal bicarbonate reabsorption in the hypothyroid rat. Am. J. Physiol. 1979;236(5):F536–F540. doi: 10.1152/ajprenal.1979.236.6.F536. Renal Fluid Electrolyte Physiol. [DOI] [PubMed] [Google Scholar]
- 23.Narayan P, Towle HC. Stabilization of a specific nuclear mRNA precursor by thyroid hormone. Mol. Cell. Biol. 1985;5:2642–2646. doi: 10.1128/mcb.5.10.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Preisig PA, Ives HE, Cragoe EJ, Jr., Alpern RJ, Rector FC., Jr. Role of the Na/H antiporter in rat proximal tubule bicarbonate absorption. J. Clin. Invest. 1987;80:970–978. doi: 10.1172/JCI113190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Preisig PA, Rector FC., Jr. Role of Na/H antiport in rat proximal tubule NaCl absorption. Am. J. Physiol. 1988;255(24):F461–F465. doi: 10.1152/ajprenal.1988.255.3.F461. Renal Fluid Electrolyte Physiol. [DOI] [PubMed] [Google Scholar]
- 26.Ribeiro RC, Apriletti JW, West BL, Wagner RL, Fletterick RJ, Schaufele F, Baxter JD. The molecular biology of thyroid hormone action. Ann. NY Acad. Sci. 1995;758:366–389. doi: 10.1111/j.1749-6632.1995.tb24843.x. [DOI] [PubMed] [Google Scholar]
- 27.Schmit CA, McDonough AA. Thyroid hormone regulates − and + isoforms of Na-K-ATPase during development in neonatal rat brain. J. Biol. Chem. 1988;263:17643–17649. [PubMed] [Google Scholar]
- 28.Wakabayashi S, Shigekawa M, Pouyssegur J. Molecular physiology of vertebrate Na/H exchangers. Physiol. Rev. 1997;77:51–74. doi: 10.1152/physrev.1997.77.1.51. [DOI] [PubMed] [Google Scholar]
- 29.Walker P, Dubois JD, Dussault JH. Free thyroid hormone concentrations during postnatal development in the rat. Pediatr. Res. 1980;14:247–249. doi: 10.1203/00006450-198003000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Yonemura K, Cheng L, Sacktor B, Kinsella JL. Stimulation by thyroid hormone of Na+-H+ exchange activity in cultured opossum kidney cells. Am. J. Physiol. 1990;258(27):F333–F338. doi: 10.1152/ajprenal.1990.258.2.F333. Renal Fluid Electrolyte Physiol. [DOI] [PubMed] [Google Scholar]