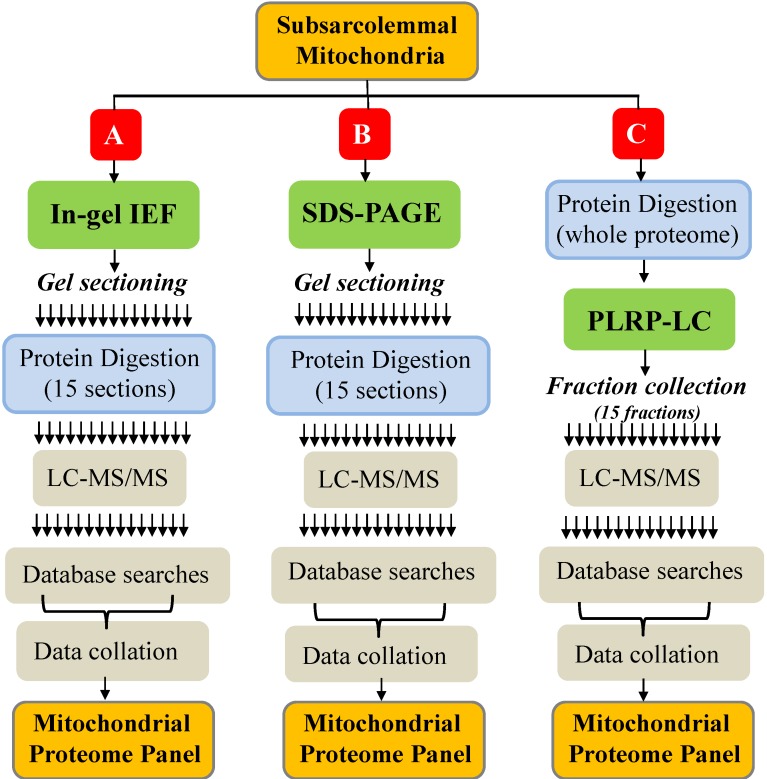
Figure 1.
Experimental scheme for the comparative evaluation of three bioanalytical platforms for mitochondrial proteome profiling. Mitochondrial protein mixtures were separated at the protein level by in-gel isoelectric focusing (IEF) (A) or sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (B); or by PLRP (polymeric reversed-phase liquid chromatography at high pH) at the peptide level following proteolytic digestion (C). Fifteen gel sections (A,B) or 15 liquid fractions (C) were produced, and the peptide mixtures were analyzed by liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS). The LC-MS/MS data were used to identify the proteins sampled with each platform. Three technical replicates were performed with each platform. Multiple arrows indicate multiple fractions/sections.