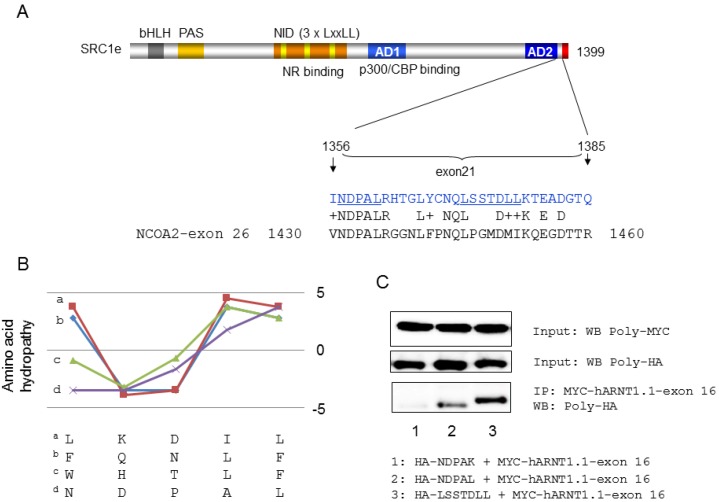
Figure 3.
Evaluation of the hARNT1.1 AF-2 binding site in SRC1e exon 21. (A) Scheme of SRC1e and amino acid composition of exon 21 (upper image) and alignment with NCOA2 exon 26. Underlined aa are the binding sites for hARNT1.1 AF-2; (B) Comparison of hydropathy pattern between C-terminal binding peptides for AF2 bindings in hAR [26] and the common NDPAL sequence in exon 21 of SRC1e and exon 26 of NCOA2; (C) Analysis of hARNT1.1 and SRC1e exon 16 interactions with immunoprecipitation of HA- tagged GFP-SRC1e-exon 21 aa stretches and MYC-tagged GFP-hARNT1.1-exon 16 with MYC-beads. Upper panel: WB of MYC-tagged GFP-hARNT1.1-exon 16 after MYC-beads immunoprecipitation with poly-MYC antibodies; Middle panel: WB of indicated HA-tagged GFP-SRC1e-exon 21 aa stretches with poly-HA antibodies (cell lysates); Lower panel: WB after MYC-beads immunoprecipitation with poly HA antibodies. Abbreviations: PAS, Per/ARNT/SIM domain; bHLH, basic helix-loop-helix domain; NID, nuclear receptor interaction domain; AD1 and AD2, activation domain 1 and activation domain 2. (Transfected HeLa cells were lysed and the supernatant was incubated with anti-myc-conjugated agarose (Sigma-Aldrich, St. Louis, MO, USA) overnight at 4 °C. The resulting precipitate was washed with RIPA buffer and then boiled in 2xSDS sample buffer for 10 min. Co-immunoprecipitated HA tagged NDPAL, NDPAK, and LSSTDLL were analyzed by Western blotting with anti-HA-polyclonal antibodies (Sigma-Aldrich, St. Louis, MO, USA) followed by horseradish-peroxidase-conjugated anti-rabbit IgG (Thermo Fisher Scientific Inc., Rockford, IL, USA) incubation for 1 h. Immunoprecipitate analysis was done using a SuperSignal West Dura chemiluminescent detection system (Thermo Fisher Scientific Inc., Rockford, IL, USA).