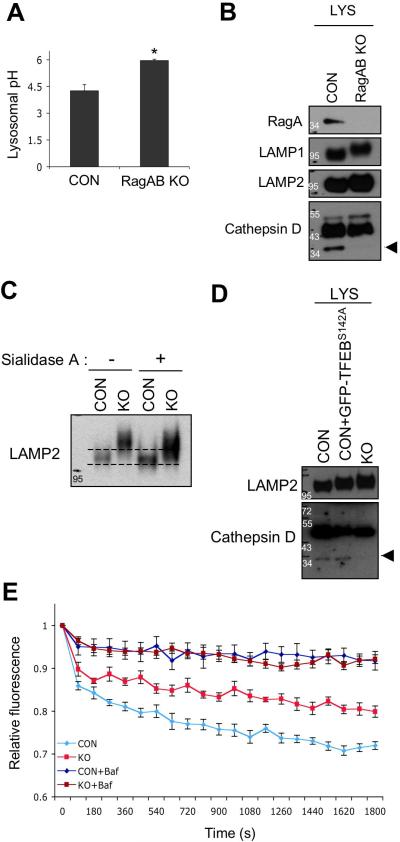
Figure 9. Lysosomal acidification is compromised in RagA/B KO MEFs.
(A) Lysosomal pH measurement in live cells. Cells were fed with OG541-conjugated dextran for overnight followed by serum-starvation for 1 hour, and then trypsinized for spectrofluorimetric measurement. Values represent the mean ± SD of data (n=3 per group). *P < 0.05, Wilcoxon rank sum test. (B) Immunoblot analysis of cathepsin D maturation in lysosome fraction. Lysosome fractions from control and RagA/B KO MEFs were analyzed by immunoblotting using the indicated antibodies. The amount of protein loading was normalized with LAMP1 protein level. Arrowhead indicates the matured form of cathepsin D. (C) Sialidase A treatment increases mobility of LAMP2 on SDS-PAGE. Cell lysates were incubated with sialidase A for 3hrs followed by SDS-PAGE and immunoblotting. (D) Cathepsin D maturation in GFP-TFEBS142A expressing cells. The amounts of matured cathepsin D in lysosomal fraction of GFP-TFEBS142A expressing cells were compared with control and RagA/B KO MEFs using immunoblotting. The amount of protein loading was normalized with LAMP2 protein level. Arrowhead indicates the matured form of cathepsin D. (E) A compromised lysosomal acidification capacity in RagA/B KO MEFs. Cells were fed with a pH sensitive fluorescent dye (OG541)-conjugated dextran for overnight, and then starved in a serum-free medium for 2 hours. v-ATPase-mediated acidification of lysosome-enriched fraction was measured in the absence or in the presence of bafilomycin A1 (100nM) in vitro. The amount of lysosome fraction was normalized with LAMP2 protein level. Each point represents the mean ± S.E of three 30-second intervals from three independent measurements. CON, control MEFs; KO, RagA/B KO MEFs; Baf, bafilomycin A1.