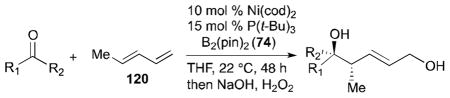
Table 6.
Ketone–diene coupling for 1,5-diol synthesis.a
| ||||
|---|---|---|---|---|
| Entry | Ketone | Product | Yield | d.r. |
| 1 |
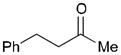 162 |
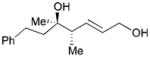 163 |
69 | 7:1 |
| 2 |
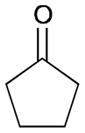 164 |
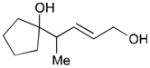 165 |
56 | N/A |
| 3 |
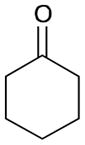 166 |
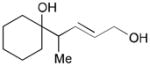 167 |
52 | N/A |
| 4 |
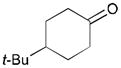 168 |
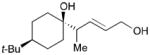 169 |
53 | >20:1 |
Reaction conditions: 10 mol % Ni(cod)2, 15 mol % P(t-Bu)3, 2.0 equiv. diene, 2.0 equiv. B2(pin)2 (74), 0.2 M THF, rt, 48 h. Then, oxidative workup with H2O2 and NaOH.
