Figure 3.
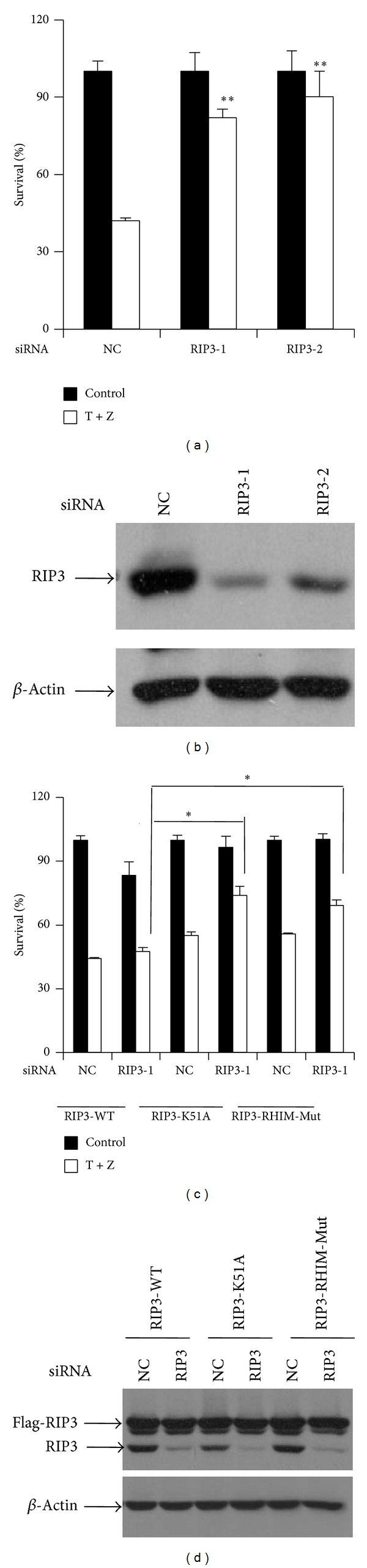
TNF-α-induced necrosis of HT-22 cells depends on RIP3 and its kinase activity. (a) HT-22 cells were transfected with the negative control (NC) or RIP3 siRNAs. After 60 h, cells were treated with control or TNF-α/z-VAD for another 20 h and then cell viability was determined by measuring ATP levels. Data were represented as mean ± standard deviation of duplicates. *P < 0.01, **P < 0.001 versus NC-T + Z. (b) The knockdown efficiency of RIP3 RNAi. Cell lysates were collected 60 h after transfection and subjected to western blot analysis of RIP3 and β-actin levels. (c) HT-22 cells stably expressing a siRNA-resistant WT-RIP3 or RIP3-K51A or RIP3-RHIM-Mut were transfected with the control or RIP3 siRNAs. After 60 h, cells were treated with control or TNF-α/z-VAD for 20 h and then cell viability was determined by measuring ATP levels. Data were represented as mean ± standard deviation of duplicates. *P < 0.01, **P < 0.001 versus NC-T + Z. WT-RIP3: HT-22 cells stably expressing a siRNA-resistant wild-type form of RIP3; RIP3-K51A: HT-22 cells stably expressing a siRNA-resistant RIP3 kinase dead mutant. RIP3-RHIM Mut: HT-22 cells stably expressing a siRNA-resistant RHIM domain mutant form of RIP3. (d) The knockdown efficiency of RIP3 RNAi. Cell lysates were collected 60 h after transfection and subjected to western blot analysis of RIP3 and β-actin levels. All experiments were repeated three times with similar results.
