Abstract
With global temperatures projected to surpass the limits of thermal tolerance for many species, evaluating the heritable variation underlying thermal tolerance is critical for understanding the potential for adaptation to climate change. We examined the evolutionary potential of thermal tolerance within a population of chinook salmon (Oncorhynchus tshawytscha) by conducting a full-factorial breeding design and measuring the thermal performance of cardiac function and the critical thermal maximum (CTmax) of offspring from each family. Additive genetic variation in offspring phenotype was mostly negligible, although these direct genetic effects explained 53% of the variation in resting heart rate (fH). Conversely, maternal effects had a significant influence on resting fH, scope for fH, cardiac arrhythmia temperature and CTmax. These maternal effects were associated with egg size, as indicated by strong relationships between the mean egg diameter of mothers and offspring thermal tolerance. Because egg size can be highly heritable in chinook salmon, our finding indicates that the maternal effects of egg size constitute an indirect genetic effect contributing to thermal tolerance. Such indirect genetic effects could accelerate evolutionary responses to the selection imposed by rising temperatures and could contribute to the population-specific thermal tolerance that has recently been uncovered among Pacific salmon populations.
Keywords: aerobic capacity, climate change, egg size, evolutionary potential, heart rate, maternal effects
1. Introduction
Climate change is projected to have widespread impacts on biodiversity [1], with rising temperatures being of particular concern owing to the pervasive effects of temperature on organisms [2]. Macrophysiological studies have projected that, in the absence of adaptive responses, temperatures will surpass the limits of thermal tolerance for many species and consequently drive extinction or extirpation [3,4]. Indeed, there is growing evidence that evolutionary adaptations to climate change will be key for the long-term viability of populations [5]. The evolutionary potential of populations to adapt to change depends on the amount of existing genetic variation for environmental tolerance as well as the extent to which it is heritable and can thus respond to natural selection [6]. A powerful means of describing evolutionary potential is using quantitative genetic breeding designs to partition phenotypic variation into additive (i.e. heritable) and non-additive genetic effects [7]. However, using only these direct estimates of heritability can underestimate evolutionary potential owing to the presence of indirect genetic effects [8]. Indirect genetic effects occur when a trait is influenced by heritable traits expressed in the environment (i.e. in an interacting individual). Because the genes influencing the focal trait are expressed in other individuals, these effects act indirectly and provide heritable variation on which selection can act [9]. Indirect genetic effects are largely attributed to heritable maternal effects [10,11], which usually occur as a result of egg provisioning. In fishes, for example, maternal effects are known to contribute to a wide range of traits among offspring, including larval survival [12], stress response [13] and metabolic enzyme activity [14], suggesting indirect genetic effects may be important to the evolutionary dynamics of populations.
In aquatic ectotherms such as fish, upper temperature tolerance has traditionally been measured using the critical thermal maximum (CTmax), defined as the temperature at which an individual loses equilibrium and a righting response [15]. While CTmax represents a functional collapse of the animal, its ecological relevance is questionable, because organ systems key to fitness-promoting activities (e.g. predator avoidance, growth) likely decline before CTmax is reached [16]. In its place, the oxygen- and capacity-limited thermal tolerance framework offers a functional understanding of how temperature limits organisms in the wild. It attributes the limits of thermal tolerance to the loss of aerobic scope (i.e. the difference between minimum and maximum oxygen consumption rates) [17]. As temperature rises above an animal's optimal temperature for aerobic scope (Topt), the maximum capacity of the cardiorespiratory system to deliver oxygen to tissues cannot keep pace with increased oxygen demands, primarily owing to limitations on the ability to increase heart rate beyond a maximum level [18]. Aerobic scope is thereby reduced until an upper critical temperature (Tcrit) is reached, above which an animal's capacity for aerobic activity cannot exceed routine rates. Because of the lack of oxygen available for aerobic metabolism above routine needs, such loss of scope reduces the capacity for growth, reproduction and aerobic swimming, which can lead to reduced survival [19,20].
Pacific salmon (Oncorhynchus spp.) provide an excellent system for understanding the effects of climate change on fishes; their anadromous life history exposes them to pressures found in both freshwater and marine environments, whereas their ecological, economic and cultural value make their long-term viability a chief concern among stakeholders. Anomalously high river temperatures have recently been identified as a significant cause of mortality in Pacific salmon populations at both the juvenile [21] and adult stage [22]. Indeed, a collapse of aerobic scope has been empirically linked to high mortality during spawning migrations of sockeye (Oncorhynchus nerka) salmon [20]. A clear, population-specific correspondence between adult Topt and the modal temperature historically experienced during spawning migrations suggests that natural selection imposed by river conditions has shaped thermal adaptation in salmon [23,24]; however, the heritability of thermal tolerance and its evolutionary potential to respond to rising temperatures remain largely unknown.
The aim of this study was to evaluate the evolutionary potential of oxygen-limited thermal tolerance within a coastal population of chinook salmon (Oncorhynchus tshawytscha). To do so, we measured the thermal performance of juvenile cardiac function within a quantitative genetic breeding design, and partitioned the phenotypic variation into additive genetic, non-additive genetic and maternal effects. Because the thermal tolerance of Pacific salmon populations appears to be adapted to local river temperatures [23,24], we predicted that we would detect additive genetic variation for thermal tolerance.
2. Material and methods
The study population consisted of wild chinook salmon from the Big Qualicum River, British Columbia, Canada. This population is augmented by a hatchery release programme. Such programmes can increase the standing genetic variation within populations that might have low genetic diversity owing to a large decline in abundance from historic levels [25]. On 8 October 2011, adult fish completing their spawning migration were collected using diversion channels located at the Fisheries and Oceans Canada salmon hatchery on the Big Qualicum River. Only unmarked, non-hatchery raised fish were selected for the study. Prior to gamete collection, each spawner was euthanized by cerebral concussion and measured for post-orbital hypural body length (±0.1 cm). Egg and milt samples from five females and five males were collected and transported on ice to Yellow Island Aquaculture Ltd on Quadra Island, BC, where mean egg diameter was measured using 30 eggs from each female (±0.01 mm).
(a). Breeding design and offspring rearing
Gametes were crossed in a full-factorial breeding design (North Carolina II cross) [7] in which all possible crosses were conducted between five males and five females, producing 25 unique families. Fertilized eggs were incubated in a Heath stack, with all families exposed to the same thermal conditions throughout development (see the electronic supplementary material for more details). After entry into the exogenous feeding stage, hatched offspring were tagged using visible implant elastomers (Northwest Marine Technology, Shaw Island, WA, USA) and transported to the University of British Columbia in Vancouver. There, the fish were kept for the remainder of the experiment in a 1000 l tank that averaged 9.3 ± 0.7°C.
(b). Cardiac performance measurements
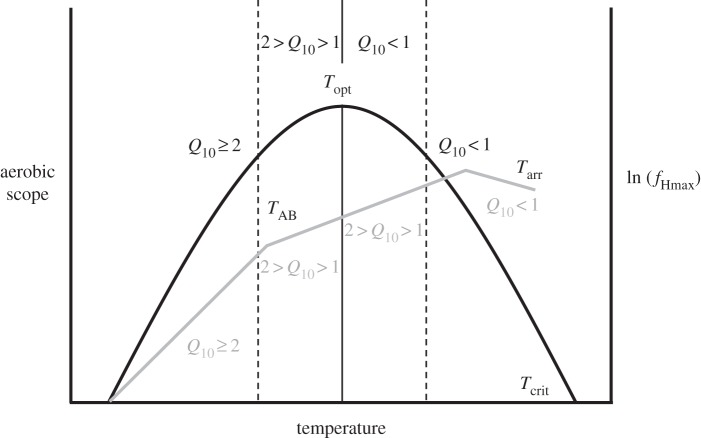
We used the response of maximum heart rate (fH) to warming [26] to evaluate the genetic architecture underlying the thermal performance of cardiac function. These measurements generate two transition temperatures—the Arrhenius break temperature (TAB) and the arrhythmic temperature (Tarr) of maximum fH (fHmax)—that provide functional indications of corresponding transition temperatures associated with a limitation in aerobic scope at and above Topt (figure 1). Increasing routine fH until fHmax is reached is the primary way in which fish supply the increased oxygen demands that occur during acute warming [18,27]. Thus, when increases in fHmax with increasing temperature start to become limited (i.e. at TAB), there should be a corresponding limitation in aerobic scope that ultimately sets Topt. Similarly, the temperature at which fHmax becomes arrhythmic should signal an approaching Tcrit, as aerobic capacity above this temperature would be highly reduced with an arrhythmic heartbeat.
Figure 1.
The relationship between aerobic scope (black line), maximum heart rate (fHmax; grey line), and temperature in Pacific salmon (Oncorhynchus spp.). Shown are the optimum temperature (Topt) and upper critical temperature (Tcrit) for aerobic scope, as well as the Arrhenius break temperature (TAB) and arrhythmic temperature (Tarr) of fHmax. The solid vertical line represents Topt, and the dashed lines indicate the optimum temperature range in which aerobic scope is ≥90% of that at Topt. Also shown are the temperature sensitivities (Q10) of aerobic scope (black) and fHmax (grey). A Q10 ≥ 2 represents an exponential increase with temperature. When fHmax becomes limited with increasing temperature (i.e. at TAB), there is a corresponding limitation in aerobic scope that ultimately sets Topt. Similarly, when high temperatures induce cardiac arrhythmia (Tarr), the capacity for aerobic activity is highly reduced, thus corresponding with Tcrit [16,26].
At their acclimation temperature of 10°C, individuals were anaesthetized in MS-222 (Sigma-Aldrich, St Louis, MO, USA) and measured for their resting fH in recirculating holding troughs (see [26] for apparatus details). fHmax was pharmacologically induced and measured at every +1°C temperature increment until the heartbeat became arrhythmic (see the electronic supplementary material for detailed methodology). A total of 228 trials were conducted using 8–10 individuals from each of the 25 families. The pharmacological stimulation occasionally had incomplete or unexpected effects on individuals, such as cardiac arrhythmias occurring soon after stimulation. In such cases, individuals were removed from the study (32 fish were removed).
(c). Thermal tolerance measurements
We measured the CTmax of offspring from each family to determine the genetic and maternal effects underlying CTmax and to assess whether these effects are similar to those underlying cardiac performance. Fish were kept in a 50 l tank at 10°C for 1 h, and then temperature was continuously increased until an associated loss of directed locomotor capacity and equilibrium was observed (see the electronic supplementary material for detailed methodology). A total of 173 individuals were sampled, using 6–10 offspring from each full-sib family.
(d). Statistical analyses
We calculated the TAB of fHmax using the program presented in [28]. This program fits two-segmented straight lines, which allowed us to identify the point at which temperature-induced increases in fHmax shift to a lower exponent. When data could not be adequately fitted by the program to reflect this change, they were manually fitted with two lines using SigmaPlot (Systat Software, San Jose, CA, USA) by comparing the residuals of all possible groupings of fHmax at high versus low temperatures. The point of intersection was calculated for the two lines of best fit to estimate the lowest Arrhenius break point, TAB. The temperature sensitivity (Q10) of fHmax was also calculated between each temperature increment using the formula (fHmax n+1/fHmax n) ^ (10/Tn+1 − Tn), whereby fHmax n is the maximum heart rate at temperature step n and Tn is the temperature at step n.
We tested for potential rearing location effects on offspring cardiac performance and thermal tolerance by using a two-way ANOVA with tray position (five levels) and cell location (16 levels) as fixed factors. We then partitioned the variation in offspring resting fH, highest fHmax reached (fHpeak), scope for fH (= fHpeak − resting fH), TAB, Tarr, thermal window between TAB and Tarr (Twin), and CTmax into additive genetic, non-additive genetic and maternal effects using a two-way restricted maximum-likelihood-based ANOVA with sire and dam identity and their interaction as random factors. Additive genetic, non-additive genetic and maternal effects were calculated following Lynch & Walsh [7] (see the electronic supplementary material for calculations). We also examined adult phenotypic correlates (female body length and mean egg diameter, male body length) of offspring performance by using linear regression with multiple Y-values for every X-value [29]. All statistical analyses were performed using SPSS 20 (IBM, Armonk, NY, USA). All means are reported±1 s.d.
3. Results
Upon entry into the juvenile stage of their life cycle, offspring survival across all families averaged 90 ± 15%. Offspring body mass averaged 0.59 ± 0.32 g at this time and increased to 3.6 ± 1.1 g during the measurements of cardiac performance and thermal tolerance. A two-way ANOVA revealed no significant effect of tray position or cell location on offspring cardiac performance and thermal tolerance (0.858 ≥ p ≥ 0.078 across all measures).
In general, fH increased with temperature from a resting fH of 71.1 ± 9.4 beats min−1 to the fHpeak of 163.3 ± 25.8 beats min−1, with fHpeak occurring at 21.2 ± 2.4°C (electronic supplementary material, figure S1). TAB averaged 15.0 ± 1.1°C among all individuals, which corresponded with the incremental Q10 decreasing from 2.5 ± 0.2 at 10.0°C to 1.9 ± 0.3 at TAB (electronic supplementary material, figure S2). Tarr averaged 22.4 ± 2.5°C and was lower than CTmax, which averaged 26.5 ± 1.0°C. Thus, the fHpeak of the average fish occurred 1.2°C before Tarr and 5.3°C before the loss of their righting response.
Larger offspring generally had enhanced cardiac capacity, with body mass being significantly and positively correlated with Tarr (Pearson's r = 0.225, p = 0.002), Twin (r = 0.253, p < 0.001), fHpeak (r = 0.177, p = 0.013) and scope for fH (r = 0.147, P = 0.041). In the sire and dam ANOVA, body mass significantly covaried with resting fH (p = 0.007), Tarr (P = 0.015) and Twin (p = 0.001), and was thus included in these models.
Residual, unexplained variation comprised most of the phenotypic variance for each trait; however, additive genetic, non-additive genetic or maternal effects were detected in each of the traits measured (table 1). Dam effects significantly contributed to resting fH, scope for fH, Tarr, Twin and CTmax. Conversely, sire effects significantly contributed to only resting fH. Using the sire variance component, additive genetic variance for resting fH was estimated to be 9.7 × 109 (=4 × [2.4 × 109]), representing 53% (=[9.7 × 109]/[1.4 × 1010]) of the total phenotypic variance. The dam- and sire-based variation in each of the analysed traits is shown in table 1 and the electronic supplementary material, figure S3.
Table 1.
The sire and dam effects contributing to cardiac performance and thermal tolerance in Big Qualicum River chinook salmon (Oncorhynchus tshawytscha). The results of the two-way ANOVA are summarized for resting heart rate (fH), highest fH (fHpeak), scope for fH, Arrhenius break temperature (TAB), arrhythmic temperature (Tarr), thermal window (Twin) and critical thermal maximum (CTmax). Shown are the variance components of each source (σ2), as well as the contributions to phenotypic variance (% phenotypic var) of maternal, additive genetic, and non-additive genetic effects. Significant values (p < 0.05) are italicized.
| d.f. | SS | F | p | σ2 | % phenotypic var |
||
|---|---|---|---|---|---|---|---|
| resting fH | |||||||
| dam | 4 | 3.2 × 1011 | 12.9 | <0.001 | 1.8 × 109 | maternal | 0 |
| sire | 4 | 4.5 × 1011 | 18.7 | <0.001 | 2.4 × 109 | additive | 53 |
| sire × dam | 16 | 9.4 × 1010 | 0.39 | 0.983 | 0.0 | non-additive | 0 |
| residual | 170 | 1.4 × 1010 | |||||
| fHpeak | |||||||
| dam | 4 | 1.2 × 1011 | 2.17 | 0.118 | 4.0 × 108 | maternal | 10 |
| sire | 4 | 2.2 × 1010 | 0.39 | 0.812 | 0.0 | additive | 0 |
| sire × dam | 16 | 2.3 × 1011 | 0.88 | 0.594 | 0.0 | non-additive | 0 |
| residual | 169 | 1.6 × 1010 | |||||
| scope for fH | |||||||
| dam | 4 | 1.5 × 106 | 3.13 | 0.044 | 6.7 × 103 | maternal | 22 |
| sire | 4 | 4.5 × 105 | 0.93 | 0.470 | 0.0 | additive | 0 |
| sire × dam | 16 | 2.0 × 106 | 1.06 | 0.395 | 0.0 | non-additive | 0 |
| residual | 169 | 1.2 × 105 | |||||
| TAB | |||||||
| dam | 4 | 425.2 | 0.25 | 0.909 | 0.0 | maternal | 0 |
| sire | 4 | 4064.0 | 2.34 | 0.099 | 14.39 | additive | 18 |
| sire × dam | 16 | 6969.2 | 1.44 | 0.129 | 5.54 | non-additive | 7 |
| residual | 171 | 304.9 | |||||
| Tarr | |||||||
| dam | 4 | 4.1 × 1010 | 4.32 | 0.013 | 2.2 × 108 | maternal | 29 |
| sire | 4 | 1.2 × 1010 | 1.40 | 0.311 | 2.1 × 107 | additive | 3 |
| sire × dam | 16 | 3.7 × 1010 | 0.93 | 0.540 | 0.0 | non-additive | 0 |
| residual | 168 | 2.5 × 109 | |||||
| Twin | |||||||
| dam | 4 | 468.9 | 6.28 | 0.003 | 2.69 | maternal | 39 |
| sire | 4 | 132.9 | 1.79 | 0.179 | 0.333 | additive | 5 |
| sire × dam | 16 | 296.8 | 0.85 | 0.625 | 0.0 | non-additive | 0 |
| residual | 168 | 21.49 | |||||
| CTmax | |||||||
| dam | 4 | 2.6 × 1077 | 9.79 | <0.001 | 2.0 × 1075 | maternal | 77 |
| sire | 4 | 4.4 × 1076 | 1.65 | 0.203 | 0.0 | additive | 0 |
| sire × dam | 16 | 1.0 × 1077 | 0.60 | 0.880 | 0.0 | non-additive | 0 |
| residual | 148 | 1.0 × 1076 | |||||
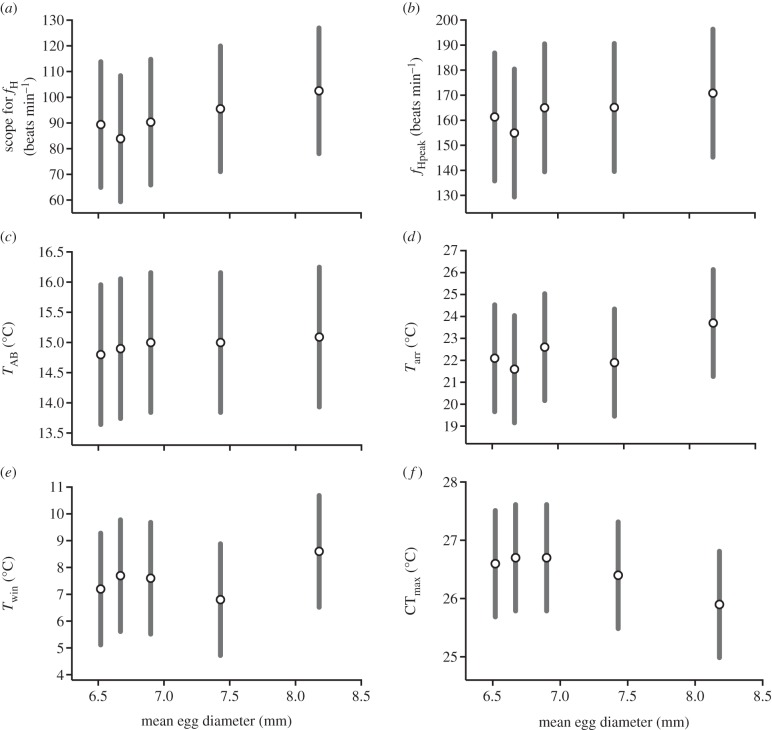
Regression analyses revealed further evidence of maternal influence on offspring phenotype, with mean egg diameter being strongly associated with scope for fH (r2 = 0.88, F1,3 = 20.3, p = 0.019) and CTmax (r2 = 0.85, F1,3 = 19.2, p = 0.022), and marginally non-significantly with TAB (r2 = 0.76, F1,3 = 9.20, p = 0.054), fHpeak (r2 = 0.70, F1,3 = 6.39, p = 0.082), Tarr (r2 = 0.59, F1,3 = 4.37, P = 0.130) and Twin (r2 = 0.51, F1,3 = 3.14, p = 0.177; figure 2). Using mass residuals of the cardiac performance traits resulted in these relationships being weaker yet still positive (data not shown), indicating that the effects of egg size were partially, but not wholly mediated by offspring body size. Conversely, no significant relationships were found across all measures for both dam body length (0.858 ≥ p ≥ 0.179) and sire body length (0.766 ≥ p ≥ 0.357).
Figure 2.
Relationships between dam mean egg diameter and offspring (a) scope for heart rate (fH); (b) highest fH (fHpeak); (c) Arrhenius break temperature (TAB); (d) arrhythmic temperature (Tarr); (e) thermal window (Twin); and (f) critical thermal maximum (CTmax) in Big Qualicum River chinook salmon (Oncorhynchus tshawytscha). The error bars are ±1 s.d. of the family mean.
There were strong phenotypic correlations between TAB, Tarr and fHpeak (Tarr and TAB: r = 0.511, d.f. = 192, p < 0.001; Tarr and fHpeak: r = 0.789, d.f. = 192, p < 0.001; TAB and fHpeak: r = 0.569, d.f. = 192, p < 0.001), whereas resting fH was significantly correlated with fHpeak (r = 0.266, d.f. = 192, p < 0.001) and Tarr (r = 0.147, d.f. = 192, p = 0.040). Using Spearman's rank-order correlations (rs) to correlate families' performance in the CTmax and fH experiments (ranked from high to low for each trait), we found highly negative correlations between CTmax and Tarr (rs = −0.541, d.f. = 23, p = 0.005), TAB (rs = −0.622, d.f. = 23, p = 0.001), fHpeak (rs = −0.563, d.f. = 23, p = 0.003) and scope for fH (rs = −0.643, d.f. = 23, p = 0.001). Indeed, the offspring of dam 1 reached the highest Tarr yet the lowest CTmax value (electronic supplementary material, figure S2d,e).
4. Discussion
Describing the genetic and environmental underpinnings of thermal tolerance is key to our understanding of how populations might respond to climate change [30]. The findings presented here comprise one of the first quantitative estimates of the genetic variation underlying thermal tolerance in a wild fish population, and the first to do so using direct measures of oxygen-limited thermal tolerance. Within a coastal population of chinook salmon, we found strong maternal effects underlying thermal tolerance and cardiac performance. These results help elucidate the adaptive mechanisms available to fish populations that are faced with rising temperatures.
Maternal effects have been found to be key determinants of phenotypic variation among offspring in a wide range of traits and taxa, and are thus increasingly recognized as having an important role in the evolutionary dynamics of populations [10,11]. For example, a study of 17 life-history and fitness-related traits among wild chinook salmon populations found that maternal effects contribute more to phenotypic divergence between populations than do additive genetic effects [31]. In our study, we detected a maternal influence on offspring thermal tolerance that far exceeded the direct influence of parental genes, with females with larger eggs having more thermally tolerant offspring. When maternal effects are themselves heritable, populations can still respond to natural selection via indirect genetic effects [9]. Indeed, in a captive population of chinook salmon, a mother–daughter regression revealed egg mass to be highly heritable, indicative of a genetic basis for egg provisioning [32]. Although heritability is population- and environment-specific, within-population variation in egg size is common in demersal egg-laying species such as salmon [33] and has been found to be similarly heritable in other species of Pacific salmonids [34,35]. If female salmon inherit the ability to provision eggs, then these maternal effects would increase the ‘total heritability’ of thermal tolerance and could accelerate any evolutionary response to the selection imposed by rising temperatures. Furthermore, this indirect genetic effect could contribute to the population-specific thermal tolerance uncovered across a number of Pacific salmon populations [23,24,36]. While the correspondence between thermal tolerance and environmental conditions suggests local adaptation brought about by selection on additive genetic effects, our study suggests that an indirect genetic effect—mediated by egg size—could instead underlie the variation. Indeed, across many sockeye salmon populations, egg size is population-specific and positively correlated with natural incubation temperatures [37] and juvenile thermal tolerance [36].
Heart rate varies considerably both within and between fish species, with resting fH being primarily determined by metabolic rate and haemodynamic requirements, and fHmax being limited by mechanistic constraints such as pacemaker potential, excitation–contraction properties and myocardium structure [38]. Ultimately, resting fH and fHmax are ‘set’ by a balance between these mechanistic constraints and the evolutionary pressures created by haemodynamic and oxygen requirements. We found that additive genetic effects account for a significant amount of intraspecific variation in resting fH, but not fHmax in juvenile chinook salmon. These differences could be owing to stronger selective pressures on maximum rates of oxygen uptake than on resting rates. Indeed, the upper limit for fH is about 120 beats min−1 across many species of adult ectothermic vertebrates [39], suggesting selection has increased maximum cardiac capacity as much as possible given common mechanistic constraints. We measured fHmax using pharmacological stimulation and acute increases in temperature, whereas resting fH was measured in anaesthetized fish at their acclimation temperature. Whether the high levels of genetic variation for resting fH still exist in high temperatures—when aerobic scope is reduced—should be investigated; indeed, individuals that can maintain a greater scope for aerobic performance by having lower resting oxygen demands might be selected for as temperatures rise, thereby allowing evolutionary adjustments of thermal tolerance.
The loss of righting response used to estimate CTmax could be caused by any effect of temperature that impairs neuronal or skeletal muscle function. A switch from aerobic to anaerobic metabolism occurs in fish as temperatures approach their CTmax [16], typical of animals experiencing hypoxic conditions. Indeed, CTmax and hypoxia tolerance are positively correlated among families of Atlantic salmon (Salmo salar) [40], suggesting a genetic basis for anaerobic capacity. We found that juvenile chinook salmon reach their maximum heart rate (i.e. fHpeak) 1.2°C cooler than their arrhythmic temperature (Tarr) and 5.3°C cooler than their CTmax. Thus, the hearts of chinook salmon begin to collapse at cooler temperatures than the whole animal, meaning their ability to aerobically avoid predation, forage or grow wanes well before CTmax. Such mismatch between tissue function and loss of righting response questions the functional and perhaps ecological utility of CTmax beyond that as a simple measure of relative thermal tolerance among groups of organisms [15]. Moreover, because fHmax is an aerobic trait owing to the heart's inability to work anaerobically at maximal levels [41], the negative correlation between CTmax and cardiac capacity among families suggests a trade-off between aerobic and anaerobic capacity consistent with earlier suggestions [42,43].
Our evidence for maternally mediated indirect genetic effects underlying thermal tolerance adds to the growing body of evidence for such ‘heritable environmental’ effects having important roles in the evolutionary potential of populations [10,11] and highlights the need for these effects to be quantified in studies of potential evolutionary responses to climate change. Indeed, with increasing evidence for parental influences on offspring environmental tolerance beyond those directly owing to the genes inherited by offspring [44], indirect genetic effects appear to be a promising source of evolutionary potential in natural populations challenged by a changing environment.
Supplementary Material
Acknowledgements
We thank A. Heath and the staff at Yellow Island Aquaculture Ltd for their invaluable support with fish husbandry, A. Berchtold for assisting the heart rate experiment, B. Coristine and the staff at the Fisheries and Oceans Canada Big Qualicum River salmon hatchery for their help with gamete collection, T. Hain for valuable discussions, and K. Gradil, R. Breckels, and two anonymous reviewers for their comments on the manuscript.
Data accessibility
The heart rate and CTmax measures from all individuals used in this study: dryad doi:10.5061/dryad.682ns.
Funding statement
This study was supported by Discovery grants to B.D.N. and A.P.F. from the Natural Science and Engineering Research Council of Canada. A.P.F. holds a Canada Research Chair in Fish Physiology, Culture and Conservation. All experiments followed ethical guidelines from the Canadian Council on Animal Care as reviewed and approved by the Animal Use Subcommittees at Western University (protocol no. 2010-214) and the University of British Columbia (protocol no. 810-022).
References
- 1.Thomas CD, et al. 2004. Extinction risk from climate change. Nature 427, 145–148. ( 10.1038/nature02121) [DOI] [PubMed] [Google Scholar]
- 2.Dillon ME, Wang G, Huey RB. 2010. Global metabolic impacts of recent climate warming. Nature 467, 704–706. ( 10.1038/nature09407) [DOI] [PubMed] [Google Scholar]
- 3.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672. ( 10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sinervo B, et al. 2010. Erosion of lizard diversity by climate change and altered thermal niches. Science 328, 894–899. ( 10.1126/science.1184695) [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann AA, Sgrò CM. 2011. Climate change and evolutionary adaptation. Nature 470, 479–485. ( 10.1038/nature09670) [DOI] [PubMed] [Google Scholar]
- 6.Blows MW, Hoffmann AA. 2005. A reassessment of genetic limits to evolutionary change. Ecology 86, 1371–1384. ( 10.1890/04-1209) [DOI] [Google Scholar]
- 7.Lynch M, Walsh B. 1998. Genetics and analysis of quantitative traits. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 8.McAdam AG, Boutin S, Réale D, Berteaux D. 2002. Maternal effects and the potential for evolution in a natural population of animals. Evolution 56, 846–851. ( 10.1111/j.0014-3820.2002.tb01396.x) [DOI] [PubMed] [Google Scholar]
- 9.Wolf JB, Brodie ED, Cheverud JM, Moore AJ, Wade MJ. 1998. Evolutionary consequences of indirect genetic effects. Trends Ecol. Evol. 13, 64–69. ( 10.1016/S0169-5347(97)01233-0) [DOI] [PubMed] [Google Scholar]
- 10.Mousseau TA, Fox CW. 1998. Maternal effects as adaptations. Oxford, UK: Oxford University Press. [Google Scholar]
- 11.Räsänen K, Kruuk LEB. 2007. Maternal effects and evolution at ecological time-scales. Funct. Ecol. 21, 408–421. ( 10.1111/j.1365-2435.2007.01246.x) [DOI] [Google Scholar]
- 12.Evans ML, Neff BD, Heath DD. 2010. Quantitative genetic and translocation experiments reveal genotype-by-environment effects on juvenile life-history traits in two populations of chinook salmon (Oncorhynchus tshawytscha). J. Evol. Biol. 23, 687–698. ( 10.1111/j.1420-9101.2010.01934.x) [DOI] [PubMed] [Google Scholar]
- 13.Heath DD, Bernier NJ, Heath JW, Iwama GK. 1993. Genetic, environmental, and interaction effects on growth and stress response of chinook salmon (Oncorhynchus tshawytscha) fry. Can. J. Fish. Aquat. Sci. 50, 435–442. ( 10.1139/f93-049) [DOI] [Google Scholar]
- 14.Patterson DA, Guderley H, Bouchard P, Macdonald JS, Farrell AP. 2004. Maternal influence and population differences in activities of mitochondrial and glycolytic enzymes in emergent sockeye salmon (Oncorhynchus nerka) fry. Can. J. Fish. Aquat. Sci. 61, 1225–1234. ( 10.1139/f04-076) [DOI] [Google Scholar]
- 15.Lutterschmidt WI, Hutchison VH. 1997. The critical thermal maximum: history and critique. Can. J. Zool. 75, 1561–1574. ( 10.1139/z97-783) [DOI] [Google Scholar]
- 16.Anttila K, Casselman MT, Schulte PM, Farrell AP. 2013. Optimum temperature in juvenile salmonids: connecting subcellular indicators to tissue function and whole-organism thermal optimum. Physiol. Biochem. Zool. 86, 245–256. ( 10.1086/669265) [DOI] [PubMed] [Google Scholar]
- 17.Pörtner HO, Farrell AP. 2008. Physiology and climate change. Science 322, 690–692. ( 10.1126/science.1163156) [DOI] [PubMed] [Google Scholar]
- 18.Farrell AP. 2009. Environment, antecedents and climate change: lessons from the study of temperature physiology and river migration of salmonids. J. Exp. Biol. 212, 3771–3780. ( 10.1242/jeb.023671) [DOI] [PubMed] [Google Scholar]
- 19.Pörtner HO, Knust R. 2007. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315, 95–97. ( 10.1126/science.1135471) [DOI] [PubMed] [Google Scholar]
- 20.Farrell AP, Hinch SG, Cooke SJ, Patterson DA, Crossin GT, Lapointe MF, Mathes MT. 2008. Pacific salmon in hot water: applying aerobic scope models and biotelemetry to predict the success of spawning migrations. Physiol. Biochem. Zool. 81, 697–708. ( 10.1086/592057) [DOI] [PubMed] [Google Scholar]
- 21.Crozier LG, Zabel RW. 2006. Climate impacts at multiple scales: evidence for differential population responses in juvenile chinook salmon. J. Anim. Ecol. 75, 1100–1109. ( 10.1111/j.1365-2656.2006.01130.x) [DOI] [PubMed] [Google Scholar]
- 22.Keefer ML, Peery CA, Heinrich MJ. 2008. Temperature-mediated en route migration mortality and travel rates of endangered Snake River sockeye salmon. Ecol. Freshwat. Fish 17, 136–145. ( 10.1111/j.1600-0633.2007.00267.x) [DOI] [Google Scholar]
- 23.Lee CG, Farrell AP, Lotto A, MacNutt MJ, Hinch SG, Healey MC. 2003. The effect of temperature on swimming performance and oxygen consumption in adult sockeye (Oncorhynchus nerka) and coho (O. kisutch) salmon stocks. J. Exp. Biol. 206, 3239–3251. ( 10.1242/jeb.00547) [DOI] [PubMed] [Google Scholar]
- 24.Eliason EJ, et al. 2011. Differences in thermal tolerance among sockeye salmon populations. Science 332, 109–112. ( 10.1126/science.1199158) [DOI] [PubMed] [Google Scholar]
- 25.Neff BD, Garner SR, Pitcher TE. 2011. Conservation and enhancement of wild fish populations: preserving genetic fitness versus genetic diversity. Can. J. Fish. Aquat. Sci. 68, 1139–1154. ( 10.1139/f2011-029) [DOI] [Google Scholar]
- 26.Casselman MT, Anttila K, Farrell AP. 2012. Using maximum heart rate as a rapid screening tool to determine optimum temperature for aerobic scope in Pacific salmon Oncorhynchus spp. J. Fish Biol. 80, 358–377. ( 10.1111/j.1095-8649.2011.03182.x) [DOI] [PubMed] [Google Scholar]
- 27.Fry FEJ. 1947. Effects of the environment on animal activity. Univ. Toronto Student Biol. Ser. 55, 1–62. [Google Scholar]
- 28.Yeager DP, Ultsch GR. 1989. Physiological regulation and conformation: a BASIC program for the determination of critical points. Physiol. Zool. 62, 888–907. [Google Scholar]
- 29.Sokal RR, Rohlf FJ. 1995. Biometry. New York, NY: W.H. Freeman and Company. [Google Scholar]
- 30.Somero GN. 2010. The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J. Exp. Biol. 213, 912–920. ( 10.1242/jeb.037473) [DOI] [PubMed] [Google Scholar]
- 31.Aykanat T, Bryden CA, Heath DD. 2012. Sex-biased genetic component distribution among populations: additive genetic and maternal contributions to phenotypic differences among populations of chinook salmon. J. Evol. Biol. 25, 682–690. ( 10.1111/j.1420-9101.2012.02462.x) [DOI] [PubMed] [Google Scholar]
- 32.Heath DD, Heath JW, Bryden CA, Johnson RM, Fox CW. 2003. Rapid evolution of egg size in captive salmon. Science 299, 1738–1740. ( 10.1126/science.1079707) [DOI] [PubMed] [Google Scholar]
- 33.Einum S, Fleming IA. 2002. Does within-population variation in fish egg size reflect maternal influences on optimal values? Am. Nat. 160, 756–765. ( 10.1086/343876) [DOI] [PubMed] [Google Scholar]
- 34.Gall GAE, Neira R. 2004. Genetic analysis of female reproduction traits of farmed coho salmon (Oncorhynchus kisutch). Aquaculture 234, 143–154. ( 10.1016/j.aquaculture.2004.01.029) [DOI] [Google Scholar]
- 35.Su GS, Liljedahl LE, Gall GAE. 1997. Genetic and environmental variation of female reproductive traits in rainbow trout (Oncorhynchus mykiss). Aquaculture 154, 115–124. ( 10.1016/S0044-8486(97)00050-1) [DOI] [Google Scholar]
- 36.Chen Z, Anttila K, Wu J, Whitney CK, Hinch SG, Farrell AP. 2013. Optimum and maximum temperatures of sockeye salmon (Oncorhynchus nerka) populations hatched at different temperatures. Can. J. Zool. 91, 265–274. ( 10.1139/cjz-2012-0300) [DOI] [Google Scholar]
- 37.Braun DC, Patterson DA, Reynolds JD. 2013. Maternal and environmental influences on egg size and juvenile life-history traits in Pacific salmon. Ecol. Evol. 3, 1727–1740. ( 10.1002/ece3.555) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lillywhite HB, Zippel KC, Farrell AP. 1999. Resting and maximal heart rates in ectothermic vertebrates. Comp. Biochem. Physiol. A 124, 369–382. ( 10.1016/S1095-6433(99)00129-4) [DOI] [PubMed] [Google Scholar]
- 39.Farrell AP. 1991. Cardiac scope in lower-vertebrates. Can. J. Zool. 69, 1981–1984. ( 10.1139/z91-276) [DOI] [Google Scholar]
- 40.Anttila K, Dhillon RS, Boulding EG, Farrell AP, Glebe BD, Elliott JAK, Wolters WR, Schulte PM. 2013. Variation in temperature tolerance among families of Atlantic salmon (Salmo salar) is associated with hypoxia tolerance, ventricle size and myoglobin level. J. Exp. Biol. 216, 1183–1190. ( 10.1242/jeb.080556) [DOI] [PubMed] [Google Scholar]
- 41.Farrell AP, Stecyk JAW. 2007. The heart as a working model to explore themes and strategies for anoxic survival in ectothermic vertebrates. Comp. Biochem. Physiol. A 147, 300–312. ( 10.1016/j.cbpa.2007.01.021) [DOI] [PubMed] [Google Scholar]
- 42.Reidy SP, Kerr SR, Nelson JA. 2000. Aerobic and anaerobic swimming performance of individual Atlantic cod. J. Exp. Biol. 203, 347–357. [DOI] [PubMed] [Google Scholar]
- 43.Blake RW. 2004. Fish functional design and swimming performance. J. Fish Biol. 65, 1193–1222. ( 10.1111/j.0022-1112.2004.00568.x) [DOI] [Google Scholar]
- 44.Donelson JM, Munday PL, McCormick MI, Pitcher CR. 2012. Rapid transgenerational acclimation of a tropical reef fish to climate change. Nat. Clim. Change 2, 30–32. ( 10.1038/nclimate1323) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The heart rate and CTmax measures from all individuals used in this study: dryad doi:10.5061/dryad.682ns.