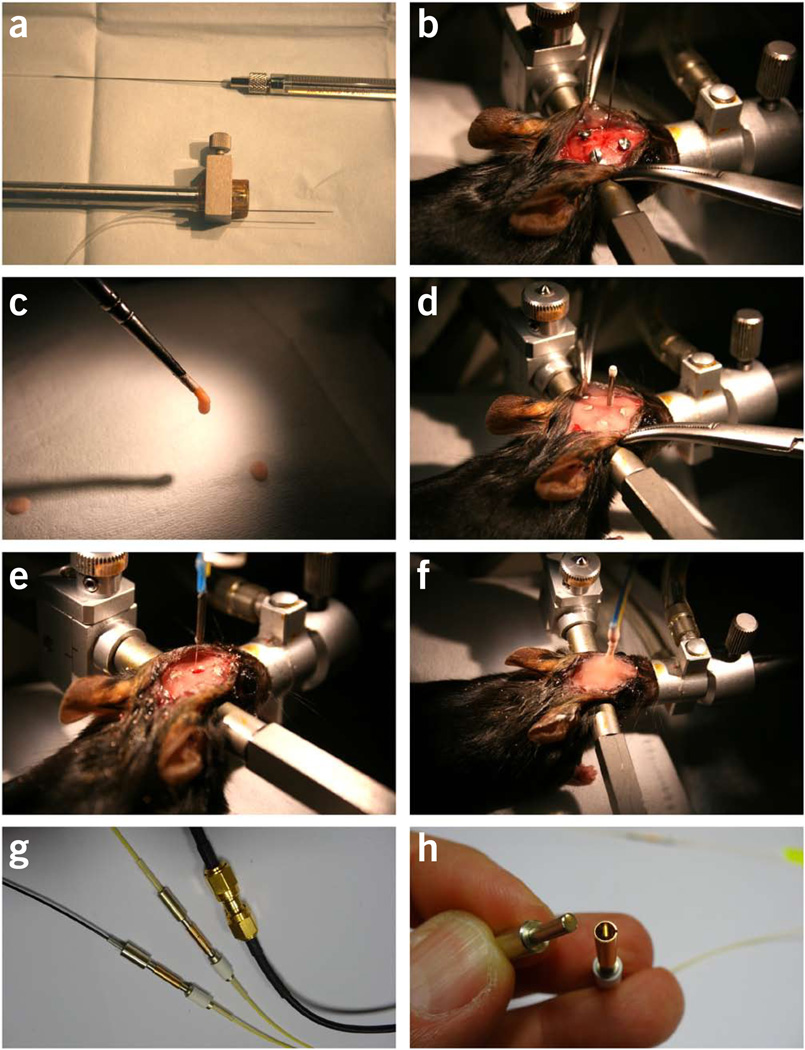
Figure 4.
Surgical procedures for intra-striatal virus injection and optical fiber implantation. (a–d) The first surgery for virus injection and creating a dental cement base. (a) A virus injection kit attached to the vertical arm of a stereotaxis. The kit consists of a 1-µl (or 2-µl) Hamilton syringe, connection polyethylene tubing (filled with saline) and a 30-gauge needle. (b) Picture showing the virus injection needle placed above the burr hole targeting the left striatum. Three anchoring screws have been driven into the skull. (c) Dental cement is applied with a paintbrush. (d) Picture showing the final step of the first surgery. A dummy cannula (marking the position of the burr hole) is fixed in place by dental cement. A thin dental cement base is created to cover the skull surface. Animals are allowed at least 2 weeks of recovery time before proceeding to next steps. (e,f) The second surgery for fiber implantation on the day of optical measurement. (e) The dummy cannula has been removed. The burr hole made during the first surgery has been re-exposed for lowering the fiber probe into the brain. (f) The final step of the second surgery. The fiber probe is fixed in place with a generous amount of dental cement. (g) Available options for connection between the implanted fibers and the fibers to the laser and the detector. (h) A detachable ferrule-sleeve connection for single-mode fibers. Panels g and h are courtesy of L. Bergann at Becker & Hickl. All animal protocols used in this study were approved by the US National Institute on Alcohol Abuse and Alcoholism Animal Care and Use Committee.