Abstract
AMPK is a serine/threonine kinase that is found in all eukaryotes and is ubiquitously expressed in all organ systems. Once activated, AMPK stimulates hepatic fatty acid oxidation and ketogenesis, inhibits cholesterol synthesis, lipogenesis, and triglyceride synthesis, inhibits adipocyte lipolysis and lipogenesis, stimulates skeletal muscle fatty acid oxidation and muscle glucose uptake, and modulates insulin secretion by the pancreas. Thus its importance in many critical cellular processes is well established. For cells it is critical that energy supply and demand are closely matched. AMPK is recognized as a critical integrator of this balance. It is known to be allosterically activated by an increased AMP:ATP ratio. Activation of the kinase switches on catabolic pathways while switching off anabolic ones. It also acts as a redox sensor in endothelial cells where oxidative stress can disturb NO signaling. Abnormal NO signaling leads to disturbed vasodilatory responses. By inhibiting the formation of reactive oxygen species in the endothelium, AMPK can optimize the redox balance in the vasculature. Here, we review the role of AMPK in the cell.
Keywords: AMPK, Cell cycle, ATP, AMP, Catabolism, Redox sensor, Review
SENSORS IN BIOLOGY
Sensors in an engineering sense are devices or components that are designed to measure a physical quantity (such as temperature or pressure) and convert that measurement into a signal that can be detected or observed by an observer or an instrument. In biology, sensors (biological sensors) operate in a manner analogous to the one described for engineered devices and different in that the input and output are biological signals. Most biological sensors are specialized cells of various types and some are intracellular molecules. Some of the many specialized cells include (a) Light: Retinal neurothelium, (b) Sound: Organ of Corti hair cells, (c) Gravity: Inner ear semi-circular canals and (d) Stretch: Carotid sinus, atrial stretch receptors, proprioceptive receptors. In addition to these larger macroscopic systems, sub-cellular sensors also operate to provide cellular systems a way of detecting changes in cellular dynamics and homeostasis and providing set points toward which dynamic corrections can be made by the cellular machinery. Some of the better studied cellular sensors include (a) pH: Regulation of a variety of protein function by changes in cell pH. Examples include alterations to substrate binding of glycinamide ribonucleotide (1), modulated protein-protein interaction of neonatal Fc receptor (2) and aggregation of P0rP octapeptide repeats (3). (b) Hypoxia: For example, the neuroepithelial bodies (NEB) found in the lungs of all vertebrates that are sensitive to changes in oxygen tension and respond by releasing bioactive substances (such as serotonin and CGRP) (4). And (c) Cell volume sensors: There are a large number of molecules that respond to an increase or decrease in cell volume and include PIP5K/PIP2, PLA2/Arachidonic acid, PI3K/PIP3, several tyrosine kinases, several ser/thr kinases and Rho GTPases (5). One of these sensor systems is the AMP-activated kinase AMPK, which is the putative metabolic or energy sensor of the cell. In recent years, another role for AMPK appears to be emerging. Endothelial cell physiology and pathology is intimately linked to the causation of many human diseases and homeostasis in these cells appears to be a central element in these disorders. Endothelial dysfunction is characterized by decreased bioavailability of endothelium-derived NO and this is paradoxically accompanied by increased expression of eNOS, together with increased ROS generation leading to the appearance and toxic effects of damaging free radical species such as peroxynitrite (6, 7). Intruigingly, it is now emerging from work done by many investigators (including our own group), that there is an intricate balance between AMPK and the cellular signaling systems that control endothelial redox balance (7). Therefore, this review will focus on AMPK’s emerging role as an ROS modulator or ROS sensor in the cell, with a particualr emphasis on the endothelium.
DISCOVERY
In relative terms, the discovery of AMPK is not recent. Its existence was uncovered by two independent observations reported in 1973 with the discovery that the same kinase inactivates 3-hydroxy-3-methylglutaryl coenzyme A (CoA) reductase and acetyl-CoA carboxylase (ACC) in hepatic fat metabolism (8, 9). Several years after these seminal reports, Munday et al, in the process of studying the Vmax of ACC, proposed the name AMP-activated protein kinase as the primary enzyme responsible for attenuating this parameter (10). Finally, with Carling et al reporting that the HMG-CoA reductase (HMGR) and ACC kinases were one and the same enzyme, the name was formally adopted in 1989 (11, 12). Subsequently, AMPK was purified and its subunit structure was analyzed by Grahame Hardie’s group at the University of Dundee (13) with detailed analysis of its all important catalytic subunit published by Bruce Kemp’s laboratory at the St. Vincent’s Medical Research Institute in Victoria, Australia (14). Based on these ground breaking studies and work my various other investigators, it has been revealed that AMPK is a heterotrimer with alpha, beta and gamma. The aalpha subunit has catalytic activity while the other two have a regulatory role. Overall, multiple AMPK subunit isoform combinations have been identified and these subunits are encoded by distinct genes. Thus far, two alpha subunits, two beta subunits and three gamma subunits have been identified (15-17).
PHYSIOLOGICAL ASPECTS OF AMPK SIGNALLING
4.1 AMPK SUBUNITS
The standardized nomenclature of AMPK subunit genes utilizes a prefix PRKA followed by the subunit identifier A1, A2, B1, B2, G1, G2 and G3 (e.g., PRKAG3) (12). The gene loci for the subunits are located on 5 different chromosomes: alpha1 (5p12), alpha2 (1q31), beta1 (12q24.1), beta2 (1q21.1), gamma1 (12q12-14), gamma2 (7q35-36) and gamma3 (2q35). With this plethora of subunits it is not surprising that gene expression and variant splicing can give rise to twelve possible heterotrimeric combinations of AMPK (18) (Figure 1).
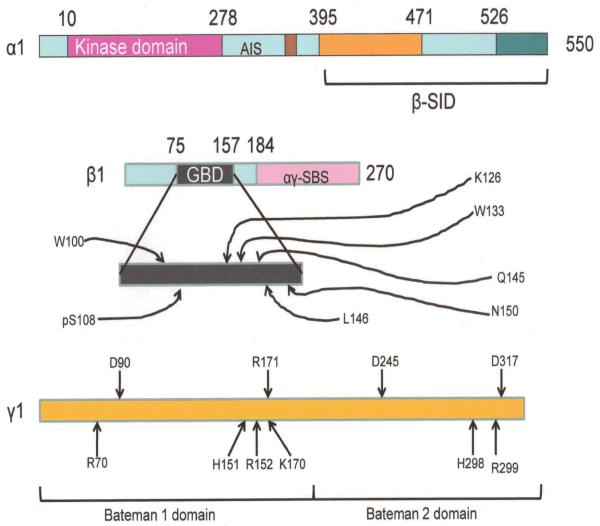
Figure 1.
Features of the AMPK subunits. Mammalian AMPK. Colored regions are ones whose structure is known. Numbers associated with α and β subunits are N- and C- terminal residues from the crystal structure. AIS: Autoinhibitory sequence. β-SID: β-subunit interacting domain. GBD: Glycogen binding domain. αγ-SBS: α and γ subunit interacting sequence. In the expanded glycogen binding domain schematic of the β subunits, letters with numbers are sites of sugar binding (pS108 is the site at Serine 108 where phosphorylation occurs). In the γ subunit, D90, R171, D245 and D317 are residues that form H-bonds with 2′ 3′-ribose hydroxyl gropus, while R70, H151, R152, K170, H298 and R299 represent basic residues that occupy the solvent accessible core of the subunit which makes contact with the nucleotide phosphates. Modified with permission from Steinberg and Kemp Physiol Rev 89:1025-1078.
Both α subunits are similar in that they both have about 550 residues (fig. 1) and both have conserved NH2-terminal catalytic domains. The beta subunits differ in the first 65 residues but in all other respects are highly conserved. The gamma subunits on the other hand (and in contrast to the other two), differ in length (gamma1 being the shortest at 331, gamma3 intermediate at 489 and gamma2 the longest at 569) (12). However, all three share a COOH-terminal having about 300 residues. Significant differences exist in AMPK subunit structure and genetic sequence between mammals and yeast, for example (multiple alpha and gamma subunits in mammals and 2 rather than three beta subunits in contrast to yeast).
Evidence suggests that variance of the alpha subunits determines subcellular localization of the molecule with the alpha1 isoform being largely cytosolic as well as being associated with the plasma membrane in carotid body type 1 cells and airway epithelial cells (19, 20). In contrast alpha2 appears to be concentrated in the nuclei of several cell types such as pancreatic beta cells, neurons and skeletal muscle (21-23).
The beta subunits feature the glycogen binding domain (GBD) which occupies a position on central conserved region of the subunit. The crystal structure of the GBD was reported in 2005 (24, 25). Another conserved region on this subunit is in the C-terminal region and there is compelling evidence that the C-terminal domain is all that is needed form a functional alpha/beta/gamma unit that can be regulated by AMP (26).
The three gamma subunits have variable N-terminal regions followed by four tandem repeats of a 60-aa sequence named as a CBS (cystathionine beta-synthase) motif by Bateman et al (27). It has since been discovered that these are actually two domains on the subunit (fig 1; Bateman 1 and 2 domains), each with the capacity to bind AMP with a 1:1 stoichiometry (28). The critical nature of these domains was revealed when investigators reported attenuated AMP binding and activation when mutations were induced in these regions (28). The Bateman domains also bind ATP antagonistically, but with a lower affinity than that for AMP (28) and this is consistent with the fact that ATP antagonizes activation of AMPK by AMP (29). Interestingly, the two Bateman domains also act cooperatively in that the second site remains inaccessible to AMP until the nucleotide has bound to the first (28). This synergy between the two domains is a potential mechanism by which AMPK activation can respond to even small changes in cellular AMP levels (18).
4.2 AMPK ACTIVATION
Mammalian AMPK is sensitive to the AMP:ATP ratio. It is therefore activated as a consequence of any cellular process, normal or anomalous, that either decreases ATP levels, or increase AMP concentrations (Figure 2). For example, mechanisms such as hypoxia, glucose deprivation or metabolic inhibition of ATP synthesis, will all activate AMPK (30). If ATP production remains unaltered but consumption is increased, the same result will ensue. Examples of increased ATP synthesis include activation of motor proteins, activity of ion channels/pumps and utilization by biosynthetic pathways. In addition, less well understood yet empirically established modulators of AMPK activity have appeared in literature and the list is ever growing. Such modulators include cytokines {leptin, adiponectin, ghrelin, cannabinoids, IL-6 (31), ciliary neutrotrophic factor [CNTF; (32)]}, certain drugs [metformin (33), thiazolidinediones (34)] and some plant derived compounds [berberine (35), resveratrol (36)], to name a few (Figure 3).
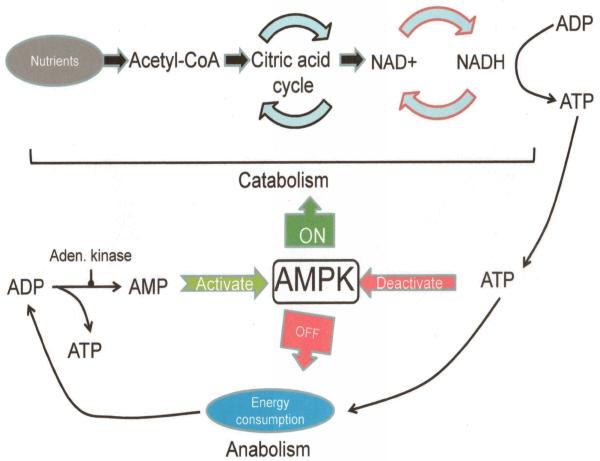
Figure 2.
Activation of AMPK by AMP:ATP ratio and influence of activated AMPK on anabolic and catabolic cellular processes.
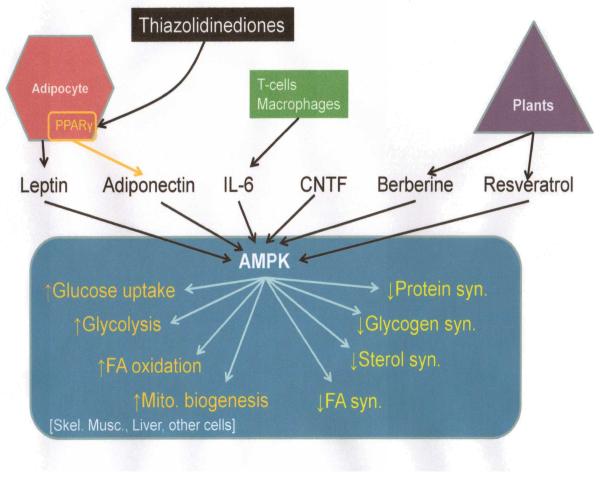
Figure 3.
Cellular effects of AMPK activation. A variety of triggers can activate AMPK that include adipocyte derived hormones like adiponectin, cytokines like IL-6, CNTF (ciliary neurotrophic factor) and plant derived modulators like resveratrol. Downstream effects are schematically outlined in typical target organs. Modified with permission from Hardie DG Nat Rev 2007.
Activation of AMPK complexes that contain the alpha1 subunit isoform are reported to be localized in the cytosol. In contrast, AMPKalpha2 activation results in translocation to the nucleus and one assumes that this occurs to facilitate modulation of gene expression (7, 37). The beta subunit also appears to be involved in determining the localization fate of the molecule in that myristoylation of the beta subunit targets the complex to the Golgi while phosphorylation on various residues promotes nuclear translocation (38).
Once AMPK is activated (Figures 3 & 4), it switches on (the concept of the “metabolic master switch”; most likely first used in the context of AMPK by Prof. Hardie of the University of Dundee) catabolic pathways that can generate ATP (e.g., cellular uptake and utilization of glucose; fig. 2) while at the same time, terminates processes that consume ATP (e.g., cellular synthesis pathways; fig. 2). The rapid “switching” required to closely and quickly regulate and balance cellular energy resources is achieved by brisk phosphorylation of metabolic enzymes as well as that of various transcription factors and co-activators which control gene expression (30, 39). The activation sequence of AMPK possesses fascinating details. It is now known that the nucleotide AMP, allosterically binds to and activates the γ subunit of AMPK which triggers phosphorylation of the alpha subunit at Thr172 (Figure 4).
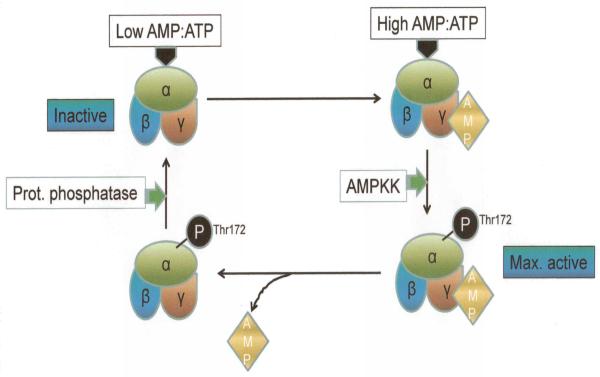
Figure 4.
Activation-deactivation cycle of AMPK. Activation of AMPK is initiated by a high AMP:ATP ratio and is triggered by AMP binding to the γ subunit and phosphorylation of Thr172 of the α subunit. This is catalyzed by AMPK kinase. The reverse (deactivation) occurs when protein phosphatase dephosphorylates the α subunit, returning AMPK to a a basal, inactive state.
4.3 UPSTREAM AMPK KINASES
Up until the early years of the 2000s decade, it had been clear that a critical phosphorylation event took place on the α subunit (on Thr172) in the process of AMPK activation (Figure 4). However, the identities of upstream phosphotransferases that were responsible for this had remained elusive. In 2003, breakthrough discoveries in the yeast system (Schizosaccharomyces pombe) identified Sak1 (Snf1-activating kinase-1), Elm-1 (elongated morphology-1) and Tos3 (Target of Sbf3) as kinases upstream of the Snf1 complex (homolog of the serine/threonine protein kinase found in S cerevisiae) (30, 40, 41). Although unequivocal human orthologs of these three kinases have not been found in the human genome, the two protein kinases closest in sequence to these are LKB1 (a serine/threonine tumor suppressor kinase) and the Ca2+/calmodulin-dependent protein kinase kinase beta (CaMKKbeta) (Fisslthaler and Fleming 2009). Evidence now demonstrates phosphorylation of the AMPK alpha subunit can either be dependent on, or independent of, its LKB1 activity. Specifically, LKB1 appears to be critically involved in the activation of AMPKalpha2 but not AKPKalpha1 (7, 41). AICAR (5-Aminoimidazole-4-carboxyamide ribonucleoside; an important activator of AMPK) is an adenosine analog taken up by muscle and phosphorylated to form 5-aminoimidazole-4-carboxamide-1-D-ribofuranosyl-5′-monophosphate (ZMP), which stimulates AMPK activity and glucose transport in skeletal muscle. LKB1 is essential for AICAR induced activation of AMPK. Empirically, it has been reported that deletion of LKB1 will prevent activation of AMPKα2 in cardiac and skeletal muscle cells (42).
Unlike LKB1, CaMKKbeta is regulated within the cell and its levels increase in response to elevations in intracellular Ca2+ ([Ca2+]i). Therefore, stimuli that amplify ([Ca2+]i (such as bradykinin and thrombin), also activate AMPKalpha1 consequent upon increased CaMKKbeta activity (43, 44).
4.4 DOWNSTREAM TARGETS OF AMPK
Once activated, AMPK can influence several downstream targets in the cell. Many of these targets are currently recognized [~20; (18)], however more are being discovered and it has been speculated that this number may eventually rise into the hundreds. These downstream effectors of AMPK influence diverse cellular processes and include lipid metabolism [e.g., acetyl-CoA carboxylase (ACC); HMG-CoA reductase], carbohydrate metabolism (e.g., glycogen synthase; 6-phosphofructo-2-kinase); cell signaling [e.g., endothelial NO synthase (eNOS); insulin receptor substrate-1 (IRS-1)], ion transport [cystic fibrosis transmembrane conductance regulator (CFTR)] and transcription [e.g., p300; hepatocyte nuclear factor-4α (HNF4-alpha); transducer of regulated CREB activity 2 (TORC2)] (16, 19, 45-52). One of the most intriguing questions has been: How does AMPK recognize its downstream targets? AMPK has been found to phosphorylate a serine residue in these targets. Further, phosphorylation sites appear to have conserved motifs where hydrophobic residues are found 5 residues from the N terminal and 4 from the C terminal (P−5 and P+4). This motif is designated phi-[beta.X]-X-X-S/T-X-X-X-phi, where phi is hydrophobic and beta is basic. This motif has been confirmed using variant synthetic peptide substrates (53, 54).
METABOLIC EFFECTS OF AMPK ACTIVATION
The primary forms of carbohydrate storage in eukaryotes are glycogen and starch. AMPK plays a key role in the interconversion of glucose (the primary cellular energy substrate) and its storage forms, by affecting the transcription and translocation of the GLUT4 glucose transporter, glycogen synthesis, glycolysis and gluconeogenesis.
The uptake of glucose across the plasma membrane via GLUT4 is regarded as the rate limiting step in glucose uptake by cells. Translocation of the transporter to the membrane is therefore a critical element in glucose utilization. The fact that AMPK activation leads to GLUT4 translocation is generally irrefutable. The signaling sequence that is involved in this translocation secondary to AMPK activation is an intensely studied topic. AMPK is now thought to directly phosphorylate AS160 [a Rab GTPase-activating protein (GAP)], which then binds to 14-3-3 proteins, an event that controls the recycling of GLUT4 vesicles (55). In addition, in skeletal muscle, the chronic elevation of AMPK by AICAR has been shown to increase GLUT4 (56). The process through which AMPK increases GLUT4 expression appears to involve the transcription factors MEF2A and 2D (myocyte enhancer factor) as well (57).
Glycogen (a short-term energy store with structural analogy to starch and amylopectin) is an important substrate for muscle tissue during exercise and for the liver during fasting (12). AMPK phosphorylates glycogen synthase (GS) at Ser7 which is a known inhibitory site for the enzyme. Young et al have also shown that AICAR-induced activation of AMPK in rat muscle cells also provoked heightened glycogen phosphorylase (GP) activity (58). Overall AMPK will therefore promote breakdown of glycogen (thereby elevating blood glucose) and suppress glycogen formation. Consistent with this idea is the fact that AMPK signaling is attenuated and muscle glycogen levels are decreased in AMPKalpha2 null mice (59).
Bergeron et al were the first to report that AMPK could inhibit hepatic glucose production in rodents (60). More convincing evidence to this effect was forthcoming from studies in AMPKalpha2 knockout mice which display hyperglycemia, glucose intolerance and increased hepatic glucose production (61). In addition, isolated hepatocytes have also shown AMPK suppression of reduced gluconeogenic gene expression when treated with metformin and adiponectin (both known activators of AMPK) (33, 62). Activation of AMPK appears to negatively regulate the transcription of gluconeogenic enzymes like L-type pyruvate kinase (L-PK), phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G-6-P) (63-65). CREB (cAMP response element binding)-regulated transcription coactivator 2 (CRTC2) is a critical regulator of gluconeogenesis (52). It has been reported that a mutation in CRTC2 (at Ser171Ala) attenuates SIK1 (salt-induced kinase 1) and AICAR mediated suppression of G-6-P, PEPCK and PGC-1alpha (Peroxisome proliferator-activated receptor gamma coactivator-1-alpha; an important regulator of mitochondrial biogenesis) (12) in primary hepatocytes. These findings suggest that CRTC2 is likely a downstream target of AMPK (as well as SIK1). In fact, it has also been found that hyperglycemia in LKB1 knockout mice is much more marked than that observed in mice with hepatic deletion of both AMPKalpha1 and alpha2 (61, 66).
When a ready supply of nutrients is not available (as might occur during fasting), lipids become the primary substrate for energy needs (67). AMPK plays a critical role in determining the fate of fatty acids through the regulation of specific substrates. Acetyl-CoA Carboxylase (ACC) is a biotin-dependent enzyme that catalyzes the irreversible carboxylation of acetyl-CoA to produce malonyl-CoA through its two catalytic enzymes, biotin carboxylase (BC) and carboxyltransferase (CT). The most important function of ACC is to provide the malonyl-CoA substrate for the biosynthesis of fatty acids (68). It was reported several years ago that AMPK phosphorylated ACC at three sites: Ser79, Ser1200 and Ser1215 (10, 69). Subsequently, using site directed mutagenesis it was confirmed that Ser79 is the physiologically relevant phosphorylation site involved in inhibition of ACC by AMPK (70). Indeed, experiments with AICAR have also confirmed the importance of Ser79 in this context (29).
Fatty acid synthase (FAS) is the enzyme responsible for de novo synthesis of fatty acids (71). It is thought to catalyze the formation of long-chain fatty acids from acetyl-CoA, malonyl-CoA and NADPH. Foretz et al have demonstrated that AMPK inhibits the glucose-stimulated transcription of FAS (72). FAS expression is more directly controlled by the transcription factor SREBP1c (sterol regulatory element binding protein 1c). Although it has been observed that when the constitutively active, truncated form of AMPK, CA-AMPK is overexpressed in the liver, SREBP1c expression is down-regulated (73), the exact mechanism behind this phenomenon is not understood. Studies using 32P labeling have also shown that both AICAR and metformin increase the incorporation of the label into FAS, further strengthening the idea that AMPK can modulate the expression of this important enzyme in the context of lipid metabolism (74).
Glycerol-3-Phosphate Acyl-Transferase (GPAT) catalyzes the initial and rate-limiting step of glycerolipid synthesis. Two distinct GPAT isoenzymes had been identified in mammalian tissues, an N-ethylmaleimide (NEM)-sensitive isoform in the endoplasmic reticulum membrane (microsomal GPAT), and an NEM-resistant form in the outer mitochondrial membrane (mtGPAT) (75). The activation of AMPK by AICAR has been reported to reduce GPAT activity in the liver (76). In addition, AMPK activation in the liver and adipose tissue in endurance athletes has also been shown to reduce the activity of mtGPAT (77). It is also intriguing to note that AMPK activation in the liver in the fasting state inhibits both GPAT and DGAT (an enzyme that catalyzes the formation of triglycerides from diacylglycerol and Acyl-CoA), and these effects are reversed by breaking the fast which effectively terminates AMPK signaling (78, 79). Therefore, the overall evidence suggests that AMPK regulation of these enzymes is likely an additional control point in this important lipid metabolism pathway.
Hormone sensitive lipase (HSL) functions to hydrolyze the first fatty acid from a triacylglycerol molecule, freeing a fatty acid and diglyceride (a process termed lipolysis). It is also known as triglyceride lipase, while the enzyme that cleaves the second fatty acid in the triglyceride is known as diglyceride lipase, and the third enzyme that cleaves the final fatty acid is called monoglyceride lipase. In the context of a role of AMPK in lipolysis, it is interesting to realize that experiments using 3T3-L1 adipocytes expressing dominant negative as well as CA-AMPK have demonstrated a role for AMPK inhibition of this process. Indeed, data from adipocytes derived from AMPKα1 knockout mice have also supported this idea (80). Furthermore, AMPK also appears to down regulate HSL activity during muscle contraction. When skeletal muscle is in the relaxed state, AICAR-induced AMPK activation also suppresses triglyceride hydrolysis (76, 81-83). Thus, AMPK activation also appears to be a negative regulator of hydrolysis of fatty acid residues.
Against the background of literature that increasingly supports the role of AMPK as “lipid status” sensor as well as a metabolic switch, several facts are known. In the heart and the liver, AMPK activation may be influenced by fatty acid availability independent of cellular AMP levels (84, 85). Clinically, reduced fat oxidative capacity has been reported in type 2 diabetics (86). In rodents, a high fat diet has been reported to significantly decrease phospho-AMPK in the liver and skeletal muscle (87, 88). It has also been observed that AMPK activity is reduced in aortic endothelium and muscle in obese rats compared with lean controls (89, 90). Overall, these studies suggest that chronic exposure to fatty acids can act to inhibit AMPK but the underlying mechanism has not been fully explained. Our group has previously examined a possible mechanistic framework previously. For example, we have evaluated the effect of the saturated fatty acid palmitate (which contributes 30-40% to the circulating plasma free fatty acid (FFA) levels) on AMPK both in vitro and in vivo. In summary, we found that in bovine aortic endothelial cells (BAEC), high concentrations of palmitate significantly suppressed AMPK and ACC phosphorylation and that the inhibition of AMPK by this fatty acid was independent of the upstream AMPK kinase, LKB1. Further, we found that PP2A (an important serine/threonine kinase that is critically involved in cell signaling systems such as those involving RAF, MEK and AKT) mediated the inhibition of AMPK by palmitate. Since ceramide is known to be a potent activator of PP2A, we tested to see if this sphingolipid was also involved in mediating palmitate-induced inhibition of AMPK. In fact we found that palmitate appeared to inhibit AMPK be increasing the synthesis of ceramide. Finally, we also discovered that mice fed with a palmitate-based high fat diet were not only prone to obese in comparison with controls, but also developed insulin at 3 months of age (91). Our laboratory was also able to demonstrate another intriguing and rather more indirect relation between AMPK activity and fatty acid metabolism. In trying to uncover mechanism at work behind the phenomenon of weight loss in chronic smokers and subsequent weight gain in these individuals if they quit smoking, we observed that nicotine (a principal addictive constituent of tobacco smoke), could increase the phosphorylation of AMPK, ACC and LKB1 in adipocytes and that the activation of AMPK was dependent on oxidative stress provoked by nicotine. Interestingly, we also found that nicotine inhibited fatty acid lipase (FAS) activity in this cell type. In fact, AMPK activation (or constitutive activation) inhibited FAS activity in adipocytes and this inhibition was affected by phosphorylation of the enzyme. We concluded that the anti-lipogenic effects of nicotine are manifest, in part, from an induction of AMPK phosphorylation (at Thr172) and LKB1 (at Ser428) (74).
AMPK AND MITOCHONDRIAL BIOGENESIS
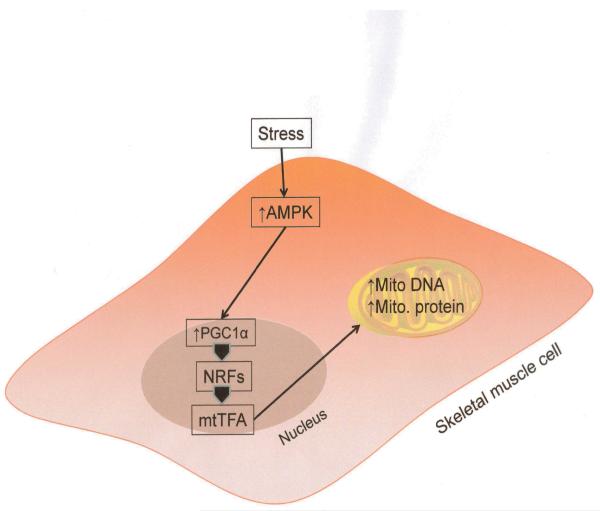
Mitochondrial biogenesis is the process by which new mitochondria are formed in the cell. This complex process is activated by numerous signaling elements, typically triggered in cellular stress or in response to environmental stimuli. It is thought that higher mitochondrial copy number (or higher mitochondrial mass) is protective for the cell. Thus, it has been reported that mitochondrial density is critically reduced in the face of insulin resistance and lipid accumulation in muscle tissue (92). Historically, Winder et al were the first to report a connection between AMPK and mitochondrial biogenesis when they reported that chronic AICAR treatment stimulated this process in skeletal muscle (93). Several different transcription factors and coactivators appear to be involved in controlling mitochondrial biogenesis. NRF-1 and -2 (nuclear respiratory factor 1 and 2) transcriptionally control the genes that encode for all the five electron chain complexes in the mitochondrion (94). Therefore it is interesting to note that NRF expression is increased by chronic AMPK activation, such as that occurs with beta-GPA (beta-guanidopropionic, a creatine analogue) (60). Another important mitochondrial biogenesis regulator is the inducible coactivator of nuclear receptors, PGC1alpha (PPAR-gamma-coactivator 1alpha). This coactivator is increased in response to activation by AMPK and is reduced in AMPKalpha2 null mice as well as AMPKalpha2 dominant negative animals (77, 95, 96). More recently, it has also been reported that PGC1alpha coimmunoprecipitates with AMPKalpha2 and that AMPK can directly phosphorylate PGC1alpha on Thr177. Kukidome et al and others have also shown that AICAR increases mRNA expression of NRF-1 and mitochondrial DNA transcription factor A (mtTFA) and stimulates mitochondrial proliferation (97) (Figure 5). Thus AMPK also lives up to its reputation as a “master switch” in that it can potently control organellar biogenesis in response to cell stress or high metabolic demand.
Figure 5.
AMPK activation signaling that leads to mitochondrial biogenesis. Activated AMPK signals via Peroxisome proliferator-activated receptor gamma, coactivator 1 alpha (PGC-1α), nuclear respiratory factors (NRFs) and mitochondrial transcription factors (mtTFA) to express genes encoding for mitochondrial DNA and proteins in stressed conditions. This leads to increase in mitochondrial density.
ROLE OF AMPK IN CELLULAR SYNTHETIC FUNCTION
HMG-CoA reductase (or 3-hydroxy-3-methyl-glutaryl-CoA reductase or HMGR) is the rate-controlling enzyme of the mevalonate pathway, the metabolic pathway that produces cholesterol and other isoprenoids. The catalytic activity of HMGR is inhibited by phosphorylation on Ser872 by AMPK (12, 98, 99). Further, HMGR is also inhibitable by ATP depletion. In transfected cells, mutation in HMGR Ser871Ala creates insensitivity to AMPK inhibition((12). Finally, AMPK can also be activated by adiponectin and this can reduce cholesterol synthesis and consequent atherosclerotic lesions in ApoE-deficient mice (100, 101). These data compellingly suggest that HMGR regulation by AMPK is critically involved in cholesterol metabolism.
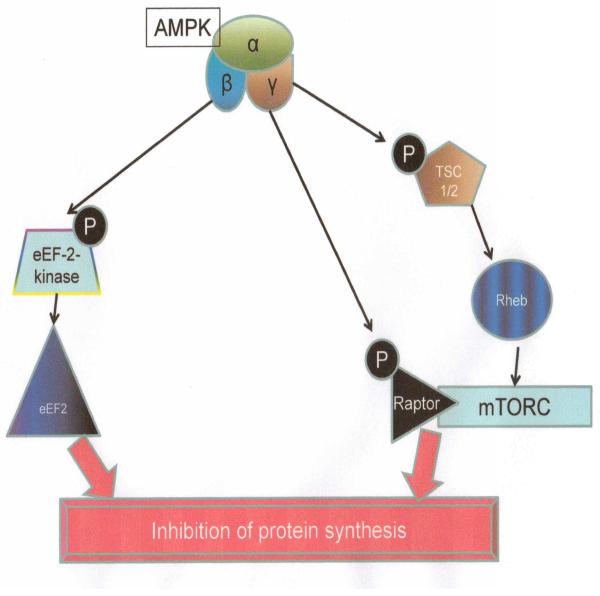
Given that protein synthesis by the cellular machinery is such an energy intensive process, it is not surprising that AMPK is intrinsically involved in this area of cell function as well. It effectively inhibits protein synthesis at multiple points (Figure 6). First, it phosphorylates and activates the eukaryote elongation factor 2 kinase (eEF-2-kinase). This phosphorylation causes phosphorylation of eEF2 (eukaryotic elongation factor 2) which in turn leads to inhibition of protein synthesis (102). Second, AMPK also inhibits the mammalian target of rapamycin complex (mTORC). While insulin and IGF-1 can promote protein synthesis by activating mTORC, AMPK counteracts this effect in various tissues. It appears to do this by: (i) Phosphorylation of TSC2 (tuberous sclerosis gene 2) at Ser1387. This amplifies the activity of Rheb (Ras homolog enriched in brain) and in turn leads to inhibition of mTORC1 signaling, and (ii) AMPK also inhibits mTORC by directly phosphorylating Raptor (a binding partner on mTORC) which causes Raptor binding to 14-3-3 proteins and ultimately, to inhibition of mTORC as well (103-105).
Figure 6.
Inhibition of protein synthesis by AMPK. eEF2 kinase: Eukaryotic elongation factor 2 kinase. TSC1/2: Tuberous sclerosis complex 1-2. Rheb: Ras homolog enriched in brain. mTORC: Mammalian target of rapamycin complex.
ROLE OF AMPK IN CELL GROWTH AND CELL DEATH
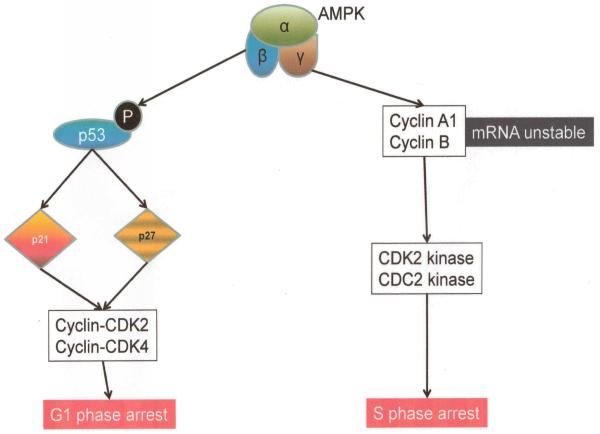
AMPK also has a far reaching impact on cell growth and proliferation and this manifests via several known mechanisms. Overall, the molecule tends to inhibit cell growth. AMPK induces G1/S phase cell cycle arrest (Steinberg 2009). Several investigators have shown that the tumor suppressor p53 and cyclin-dependent kinase inhibitors, p21 and p27, are involved in this process. The primary event is the phosphorylation of p53 at Ser15 by AMPK which occurs upstream of p21 and p27 (106-108). Furthermore, AMPK appears to be involved in reducing the cytoplasmic-to-nuclear ratio of the RNA-binding protein HuR (Hu family of RNA-binding proteins; from the rare neurological disorder, anti-Hu syndrome discovered in a patient of that name) which tends to reduce the mRNA stability of critical cell cycle regulators like Cyclin A and Cyclin B1 (109). Thus, in a complex and interactive manner, AMPK can also function to negatively regulate cell cycle progression with the final consequence of inhibiting cell growth and proliferation (Figure 7).
Figure 7.
Putative mechanism of cell cycle control by AMPK activation.
ROLE OF AMPK IN MEMBRANE EXCITABILITY AND ELECTROCHEMICAL GRADIENTS
All cells exist and function in an exquisitely regulated ionic environment where the extracellular milieu and the cellular interior are distinct in their ionic makeup. This difference has to be maintained if normal signaling between the extracellular space and the subcellular machinery can occur and, indeed for normal metabolic processes to proceed nominally. This is largely achieved through a system of ionic channels and pumps that are distributed on the plasma membrane as well as organellar surfaces inside the cell. These channels and pumps have specific properties, electrodynamic properties and kinetics. AMPK plays an important role in influencing the function of these cellular components as well. For example, AMPK has been shown to inhibit the function of the CFTR Cl− channel (cystic fibrosis transmembrane conductance regulator) as well as that of the endothelial Na+ channel, ENaC (110, 111). In the kidney, when osmotic stress is increased, AMPK is activated in the distal nephron. This activation increases the activity of NKCC2 (Na+-K+-2Cl− cotransporter 2) thereby tending to return the osmotic environment to nominal levels (112). More recently, it has also been reported that AMPK inhibits the activity of the KCa3.1 K+ channel (a Ca2+ activated K+ channel expressed at the basal lateral membrane of many epithelial cells) (113). The caveat here is that despite these disparate effects, contrary to expectation, no evidence has been uncovered that would suggest that AMPK is involved in regulating or controlling the single most energy intensive cellular function in regard to ionic homeostasis: maintaining the resting membrane potential. However, it is possible that a more indirect impact of the molecule may be revealed with subsequent study (114).
INTEGRATIVE PHYSIOLOGICAL IMPACT OF AMPK
At the supracellular or whole organism level, studying the impact of AMPK on human physiology is perhaps more relevant than simply elucidating its subcellular role. In this context, AMPK has diverse influences and is also influenced itself by a variety of factors.
A conundrum linked to lipid metabolism is that extracellular fatty acid availability somehow triggers cellular fatty acid metabolism (114). The trigger or switch that allows this to happen has not been clearly defined. Tantalizingly, reports suggest that AMPK may be sensitive to the lipid status of the cell in a variety of tissues (84, 115) and this may be independent of the AMP-induced activation of AMPK. It further appears that fatty acid activation of AMPK is only possible when AMPKalpha 1-312 (a gamma binding site on the alpha subunit) is expressed, suggesting that activation by fatty acids requires interaction with the gamma subunits of the AMPK complex (32). Further evidence for a role of AMPK as a sensor of fatty acids in vivo comes from experiments on knockout mice that lack steroyl-CoA desaturase 1 (SCD-1; which catalyzes the biosynthesis of monounsaturated fatty acids) or those that are null for the fatty acid binding protein, AP2/MAL, demonstrating that these gene deletions provoke increased levels of AMPK in the liver and skeletal muscle which affords protection to these animals from diet-induced obesity (116-118).
By phosphorylation and activation of elongation factor 2 kinase (eEF2K) and inhibition of the mTOR signaling pathway (above), AMPK can regulate protein translation. Since both eEF2K function as well as the integrity of mTOR signaling is dependent on amino acid availability, it is reasonable to suspect that AMPK might also be regulated by amino acids. In fact, it is known that amino acids can inhibit AMPK phosphorylation and this appears to be independent of mTOR (treatment with rapamycin does not alter this inhibition) (12). Recent studies compellingly demonstrate that while amino acid inhibition of AMPK does occur in the cell, it is likely an indirect phenomenon because amino acids are known to stimulate mitochondrial glutamate dehydrogenase which leads to enhanced tricarboxylic acid cycle flux and attenuation of cellular AMP levels (119).
Appetite is controlled through a complex and multi-loop feedback system that integrates in the arcuate nucleus of the mediobasal hypothalamus. Inputs into the nucleus come from circulating nutrients (such as glucose and fatty acids) and hormonal signals (from the pancreas, adipose tissue and the gastrointestinal tract). Many studies have established that AMPK is a critical link in integrating these signals. There are several lines of evidence to support this idea. AMPK subunits co-localize with neuropeptide-Y (NPY; an important orexigenic neurotransmitter)-expressing neurons. Hypothalamic AMPK is activated by fasting and inhibited by refeeding (12, 120). If AICAR is injected into the cerebral ventricles, AMPK levels rise in the hypothalamus as does the desire to feed (121). Other anorexigenic signals (such as insulin and glucose, etc.) also suppress AMPK activity in the brain.
A very intense area of medical research has been the effort to understand the role of exercise in health and disease. It has been known for a while that lack of exercise (and obesity, perhaps as an independent variable), is a risk factor for several human pathologies ranging from heart disease and stroke to diabetes and malignancy. However, the exact mechanism that might explain these very strong associations are ill understood. One of the more remarkable discoveries in biomedical research was that AMPK was activated in exercise (most likely by skeletal muscle contraction) in humans as well as rodents (122, 123). Studies in LKB1 mice have now shown that the activation of AMPKalpha2 is dependent on LKB1 phosphorylation (124, 125). Further, both AMPKalpha1 and alpha2 expression is upregulated in the heart in proportion to intensity of exercise (126).
During exercise, AMPK can regulate increased glucose uptake by skeletal muscle (12). In fact its possible role in enhancing glucose uptake via mechanisms that are independent of insulin signaling has attracted considerable interest, especially because this is likely an important mechanism in situations where muscle is insulin-resistant (as might occur in type II diabetes). It was therefore intriguing when reports surfaced that muscle contraction, glucose uptake and AMPK activity appeared to be positively associated. However, more detailed experiments such as ones using dominant negative AMPK mutant mice and AMPKalpha2 knockouts, have revealed that glucose uptake by contracted skeletal muscle was either normal or slightly decreased (59, 127-129), the latter during tetanic muscle contraction. Overall, these reports point to the possibility of more than one signaling system being responsible for dynamic changes linking exercise associated glucose uptake, and AMPK activity.
During exercise and/or muscle contraction, evidence suggests that AMPK activity is associated with deactivation of ACC2 and malonyl CoA decarboxylase (MCD)(122, 130, 131). However, specific links between fatty acid oxidation rate and AMPK activation have not clearly been established. For example, in LKB1-deficient mice, although ACC phosphorylation in both resting and contracted conditions is reduced, the level of malonyl-CoA remain unaltered and the influence of muscle contraction on this coenzyme is somewhat weak (132). Furthermore, studies also suggest that contraction-induced increases in fatty acid oxidation are independent of AMPK activity (133). Muscle blood flow is also an important compensatory mechanism wherein increased metabolic demands of active muscle groups is readily met by increases in local blood flow which serves to enhance muscle perfusion and performance. It has been observed that AMPK-induced stimulation of muscle uptake of glucose is dependent on nitric oxide (NO; a critical vasodilatory endothelium derived signaling molecule) (134). In addition, it is has also been established that AMPK can phosphorylate eNOS (endothelium derived NO synthase) at Ser1177 in contracting human skeletal muscle (135). These finding suggest that NOS phosphorylation by AMPK in the endothelium serves to connect metabolic demand with muscle perfusion.
AMPK has many disparate influences on eukaryotic endocrine physiology which include effects on specific hormonal signaling molecules and target tissues: (1) Leptin (16 kDa protein hormone) plays a key role in regulating energy intake and energy expenditure, including appetite and metabolism. Leptin is one of the most important adipose derived hormones. It is known to increase AMPK activity in skeletal muscle (136). Furthermore, rodents who lack leptin or leptin-receptors, show decreased AMPK activity in the liver (137). Brabant et al have also demonstrated that in lean animals, this hormone causes raised AMPK activity (138). In addition, it has been observed to inhibit triacylglycerol storage and increase fatty acid oxidation in the heart in an AMPK-dependent and AMPK-independent manner (139, 140). In contrast, in the brain (hypothalamus), leptin has been shown to decrease AMPK activity thus inhibiting appetite and increasing peripheral fatty acid consumption (121, 136). (2) Adiponectin (a protein hormone that modulates a number of metabolic processes, including glucose regulation and fatty acid catabolism) activates and stimulates liver and muscle AMPK activity in vivo and in vitro, thus leading to increased glucose uptake and enhanced fatty acid oxidation (141). Adiponectin has also been shown to activate AMPK in primary rat adipocytes (101, 142). Shibata et al have reported that adiponectin can protect the heart from ischemic injury via AMPK-mediated mechanisms (143). In fact, impaired AMPK signaling in adiponectin deficient mice provokes a heart failure phenotype (144). (3) Resistin (is a cysteine-rich protein hormone/signaling molecule) secreted by adipose tissue in rodents. In other mammals (primates, pigs and dogs), resistin is secreted by immune and epithelial cells. It is known to induce insulin resistance and stimulate hepatic gluconeogenesis (141). This hormone has been shown to phosphorylate AMPK in the hypothalamus, an interesting fact that does not quite match the overall anorexigenic effect of resistin (141). Vazquez et al have further opined that ACC inactivation (secondary to AMPK activation) might represent a physiological compensatory mechanism that serves to block the deleterious effect of high malonyl-CoA in the hypothalamus from resistin-induced FAS (apoptosis-mediating surface antigen) inhibition (145). (4) Ghrelin is a hormone produced mainly by P/D1 cells lining the fundus of the human stomach and ε cells of the pancreas that stimulates appetite. This hormone has also been shown to regulate AMPK activity in the hypothalamus as well as peripheral tissues (141). Kola et al have reported that ghrelin stimulates cardiac and hypothalamic AMPK activity on one hand, while inhibits it in adipose tissue and the liver on the other (146-148). (5) Perhaps the greatest impact relevant to human disease that is attributable to AMPK, is its association with insulin signaling. Centrally, insulin manifests anorexigenic effects by inhibiting AMPK activity in hypothalamus (120). AMPK activation and subsequent increase in appetite has been noted in streptozocin-induced diabetic rats (149). In peripheral tissues AMPK also has interesting associations with insulin signaling. In adipose tissue, for example, insulin will inhibit AMPK by activating the B/Akt protein kinase complex. This leads to AMPKalpha phosphorylation at Ser485 and Ser491 and reduced phosphorylation at Thr172 (150). Both insulin and AMPK can upregulate glucose uptake by skeletal muscle via enhance GLUT1 and GLUT4 translocation to the cell membrane as well as an increase in GLUT4 transcription (56, 151). AMPK is also known to upregulate IRS1 (insulin receptor substrate 1) via an inhibition of the insulin-mTOR pathway (152, 153). Thus, in several different ways, insulin and AMPK actions are interlocked to modulate hormonal control of cellular metabolism.
ROLE OF AMPK IN HUMAN DISEASES
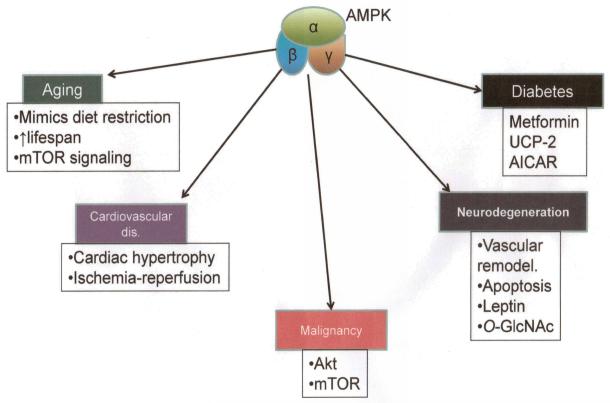
Given how far reaching the effects of AMPK are on cell function, it is not surprising that this molecule plays a central role in a variety of human pathologies and with growing interest and continued research, an understanding and appreciation of this role is expanding at a high rate (Figure 8).
Figure 8.
Role of AMPK in pathogenesis of various human disorders.
11.1. OBESITY
It is probably appropriate to begin with a description of AMPK’s role in obesity because its human health implications are so significant. Obesity is now strongly associated with diseases or disease syndromes ranging from cardiovascular diseases, diabetes mellitus type 2, obstructive sleep apnea, certain types of cancer, and osteoarthritis. Obesity is also one of the leading preventable causes of death worldwide (154). Winder et al were the first to report that AMPK activation could reduce adiposity (93). In their studies, these investigators also reported a decrease in hepatic and muscle triglyceride content. Buhl et al observed then when obese rats were treated with AICAR, intra-abdominal adipose tissue was reduced by 15% compared to pair-fed controls (155). In mice with part of the ACC2 (the dominant isoform observed in heart and skeletal muscle) gene deleted, adipose tissue was markedly reduced as was the lipid content of the liver (156). Furthermore mGPAT (mitochondrial Glycerol-3-phosphate acyltransferase; an enzyme that catalyzes the first committed step in glycerolipid synthesis and one which is inhibited by AMPK) deletion in mice gives rise to a lean body weight and reduced liver triglyceride levels (157). There is also a close link between the important anorexigenic hormone, leptin and AMPK signaling. For example, Kahn and colleagues first reported that leptin administration increased AMPK activity in skeletal muscle (136). Another important cell-derived hormone that has anorexigenic activity and is derived from adipocytes, is adiponectin. Studies a few years ago have shown that this hormone activates AMPK and attenuates ACC activity in muscle and liver, and g-adiponectin (adiponectin globular domain only) activates AMPK in adipose tissue (62, 101, 158).
11.2. AGING
The term “aging” is somewhat ambiguous. Distinctions may be made between “universal ageing” (age changes that all people share) and “probabilistic ageing” (age changes that may happen to some, but not all people as they grow older, such as the onset of type II diabetes). Thus, many investigators also make a distinction between chronological aging (age of an organism) and biological aging (changes in an organism, some of whom may be pathological). The role of AMPK in biological aging is also a hotly investigated area of research. Most fascinating is the finding in c elegans, that AMPK overexpression can increase lifespan by as much as 15% (159). Dietary or caloric restriction (DR) has long been known to extend lifespan in a variety of species across the phylogenetic scale. Anisimov et al and Ingram et al and others have opined, that AMPK activation can increase rodent lifespans by mimicking DR (160-162). AMPK signals to influence protein synthesis via the mTOR signaling system (above). Thus, TOR mutant worms also have extended lifespans as do those bearing Raptor mutations (above) (163, 164). Furthermore, modulation of translation by a dominant negative form of TOR in Drosophila, also extends lifespan (165).
11.3. MALIGNANT DISEASE
Malignant pathologies are characterized by anaplasia, invasiveness and metastases. The National Cancer Institute (NIH; Bethesda MD) have published several reviews that identify and delineate various mechanism-based targets for preventive interventions in malignant diseases. These include Akt, mTOR and epigenetic modulators (such as those affecting cell differentiation) (166). As discussed above, two of these (Akt and mTOR) are associated with AMPK activity (Akt activates mTOR signaling by inhibiting AMPK activity). Therefore, it is provocative to consider a role of AMPK in cancer biology as well.
In the simplest and most global sense, decreased AMPK activity is observed in those human metabolic disorders that are associated with high cancer risk and these include obesity, diabetes and the metabolic syndrome (166, 167). Intriguingly, even though it is not fully understood how AMPK activity contributes to these complex, multi-factorial diseases/syndromes, pharmacological intervention that activates AMPK appear to have positive therapeutic impact (168). LKB1 is an important upstream activator of AMPK. Germ-line mutations in LKB1 are linked to Peutz-Jegher syndrome (hereditary intestinal polyposis syndrome; an autosomal dominant genetic disease characterized by the development of benign hamartomatous polyps in the gastrointestinal tract and hyperpigmented macules on the lips and oral mucosa). Further, about 50% of non-small cell malignant lung tumors also show inactivating LKB1 mutations (169). Loss of LKB1 expression has also been noted in recent reports of high grade in situ ductal breast cancer (170). Evidence also demonstrates that mice lacking PTEN (phosphatase and tensin homolog; a tumor suppressor) have reduced levels of LKB1 and high AMPK activity and are protected from tumorigenesis (166). Mice that are hypomorphic to LKB1, also resist tumor formation. Indeed, pharmacologic AMPK activation can significantly delay tumor formation in PTEN+/− mice (171). p53 (a critical cell cycle regulator and tumor suppressor) expression has been observed to increase when pharmacologic AMPK activation is used in a variety of experimental settings as well as increased expression of cyclin-dependent kinase inhibitors, p21CIP and p27KIP1 (108, 172, 173). Several enzymes that are regulated by AMPK and are closely involved in cellular metabolic control have also been linked to malignant pathogenesis. These include FAS, ACC and HMGCoAR (18, 30). AICAR (widely used to activate AMPK) has been shown to inhibit growth of established tumor cell lines in vitro and include colon, breast, prostate, gastric, and glioma (108, 174). This phenomenon has also been seen in vivo in rat gliomas, human breast cancer cells and colon xenografts (108, 174, 175). In addition, AICAR has also demonstrated the ability to arrest tumor cells in S phase, increase expression of p21CIP, p27KIP1 and p53 along with inhibition of PI3K/Akt/mTOR signaling. All of these effects of AICAR have been variously confirmed as being dependent on AMPK activity (108, 174). Metformin (a biguanide activator of AMPK) has also shown some positive effects in tumor biology. For example, it has shown suppression of colon tumor xenografts in p53−/− and inhibition of estrogen receptor alpha positive breast cancer cells.
Overall, it is likely that AMPK in the activated state has a beneficial impact on solid tumor biology. However, more extensive investigation of the underlying mechanism is warranted as well as an extension of AMPK’s role in other cancerous conditions such as hemopoietic malignant pathologies and lymphomas, etc..
11.4 NEURODEGENERATION
Neurodegenerative processes underpin a diverse array of neurological pathologies. These include those where inflammation and necrosis supervene (such as neurotrauma and stroke), to those where apoptotic programmed cell death are predominant (such as Parkinson’s disease and Alzheimer’s dementia).
It is well known that AMPKalpha1 and alpha2 are highly expressed in the brain (176). AMPK in astrocytes is involved in ketogenesis and in prevention of apoptosis (177). In Alzheimer’s disease (AD), abnormal vascular remodeling has been reported and AMPK has been implicated as a contributing factor in this phenomenon (178). Interestingly, AICAR treatment has also been shown to protect neurons from injury by agents such as glucose deprivation, hypoxia, excitotoxicity and amyloid β peptide (the protein implicated in neuronal apoptosis in AD) (179). AMPKalpha2 deletion in mice has been shown to be protective in induced stroke with reduced size of infarcts (180). The adipocyte derived hormone, leptin is decreased in AD patients, and serum levels are inversely correlated to severity of dementia. Leptin has also been observed to reduce the amyloid β protein load both in vitro and in vivo, and suppress tau phosphorylation in vitro (181). In a rodent model of Huntington’s disease, treatment with metformin significantly prolongs survival time (182). In contrast to these potential benefits of AMPK activation in neurodegeneration, it has also been reported that in some conditions AMPK activity might have contrasting effects. For example, metformin, at doses that lead to activation of the AMP-activated protein kinase (AMPK), significantly increases the generation of both intracellular and extracellular Abeta species. Furthermore, the effect of metformin on Aβ generation is mediated by transcriptional up-regulation of beta-secretase [BACE1; one of the two proteolytic complexes that cleave amyloid precursor protein (APP) sequentially with gamma secretase to produce the amyloidogenic amyloid peptide], which results in an elevated protein level and increased enzymatic activity (183). A meta-analysis has also revealed that obesity and diabetes significantly and independently increase risk for AD (184). Finally, Har and colleagues have shown that a wide array of stress signals induces O-GlcNAc transferase (OGT) expression and increases O-GlcNAcylation of many intracellular proteins, a response that is critical for cell survival. His group has also described a mechanism by which glucose deprivation induces OGT expression and activity in Neuro-2a neuroblastoma cells where glucose deprivation increases OGT mRNA and protein expression in an AMP-activated protein kinase-dependent manner, whereas OGT enzymatic activity is regulated in a p38 MAPK-dependent manner (185).
11.5 DIABETES
Beginning with Hardie’s pioneering paradigm of a possible role of disturbed AMPK signaling in diabetes, a growing body of evidence has begun to validate this idea (186). In the decade of the 2000s, both clinical and basic science data has accumulated pointing to a role of AMPK signaling in type II diabetes. For example, Calvert et al have shown clinical data that suggests metformin reduces cardiovascular end points of type 2 diabetic subjects by actions that cannot solely be attributed to glucose-lowering effects. These therapeutic effects of metformin have been reported to be mediated by its activation of AMP-activated protein kinase AMPK (187). In high-fat and high-sucrose diet (HFHSD) induced diabetes in mice, Bonnard et al reported tissue-specific defects of adiponectin-receptor expression and AMPK activity (188). Several studies from our own laboratory have defined a specific role of AMPK signaling in diabetes. For example, we have shown that in human umbilical vein endothelial cells and aortas isolated from streptozocin-injected diabetic mice, AMPK activation normalizes vascular endothelial function by suppressing 26S proteasome-mediated GTPCH I degradation (189). Furthermore, when confluent HUVECs or mice were treated with AICAR for the detection of AMPK phosphorylation and the expression of mitochondrial uncoupling protein (UCP)-2, UCP-2 expression was increased, resulting in the inhibition of both O−. and prostacyclin synthase nitration in diabetes (190). In addition, Ren and colleagues have shown that the protease inhibitor 5-[5-(2-nitrophenyl) furfuryliodine]-1,3-diphenyl-2-thiobarbituric acid (UCF-101; demonstrated to protect the heart against ischemic injury), in streptozotocin (STZ)-induced diabetes, can normalize hyperglycemia and alleviates STZ-induced anomalies in cardiomyocyte contractile mechanics (191). Activation of AMPK by AICAR is rat or in muscle cells that overexpress constitutively active AMPK (CA-AMPK) can increase glucose uptake and induces translocation of GLUT1 and GLUT4 transporters (192). In AMPKalpha2 knockouts, AICAR stimulated glucose transport is blocked in muscle but insulin stimulated glucose transport is normal (127). Transgenic mice overexpressing muscle specific DN-AMPKalpha2 fail to respond to AICAR treatment in terms of muscle glucose uptake (193). Insulin resistant Zucker fa/fa rats have improved insulin sensitivity with just one dose of AICAR (194). Overall, these reports suggest that in both in vivo and ex vivo systems, AMPK mediated glucose uptake is complimentary to insulin as well as possibly independent of this hormone, thereby implicating the kinase in diabetes pathophysiology.
AMPK AND OXIDATIVE STRESS
The role of AMPK in vascular pathology is so intimately linked to endothelial function and its relationship with oxidative stress that is reasonable to study the two in a somewhat integrated manner.
CARDIAC DISEASE
The effect of AMPK activity on cardiac pathology can be considered under two broad topics. First, AMPK appears to have a role in disorders that manifest as hypertrophy of the cardiac muscle architecture. Dyck and colleagues have reported that activation of the Akt pathway is critically involved in the genesis of cardiac hypertrophy (195). Furthermore, mice lacking adiponectin that have reduced AMPK signaling are more susceptible to afterload-induced hypertrophy (196). Second, myocardial ischemia-reperfusion injury (inflammatory and oxidative damage to the cardiac muscle caused by reperfusion following a relief of ischemia), is an important cardiopathic mechanism where AMPK is also thought to play a role. It is known for example, that AMPKalpha1 and alpha2 activity is elevated during cardiac ischemia (114). Increased AMPK activity likely compensates for the ischemic condition by upregulating GLUT4 translocation and enhancing fatty acid oxidation (197, 198). However, it remains unclear if AMPK activity contributes positively or negatively to reperfusion in that reports have suggested both scenarios being dominant (199, 200).
VASCULAR ENDOTHELIAL FUNCTION
Oxidative stress and a shift in the cellular redox balance are critical underpinnings of endothelial dysfunction. In turn, endothelial disturbances underlie cardiovascular pathology (7). One important hallmark of endothelial dysfunction is a decreased bio-availability of NO, the central vasodilatory molecule that helps regulate vascular tone. The decreased availability of NO is also associated with the generation of reactive oxidative species in the vessel wall such as peroxynitrite (6). Many studies have now established that there is an intricate balance between AMPK signaling and the redox balance in the vascular milieu. For example, AMPK has been shown to inhibit the formation of reactive oxygen species (ROS) by NADPH oxidase and stimulate NO production be eNOS (endothelial nitric oxide synthase)(7). Furthermore, AMPK has also been implicated in JNK activation, NF-kappaB-mediated transcription, E-selectin expression and VCAM-1 expression, in endothelial cells that have been exposed to H2O2, TNF-alpha or fatty acids. As a consequence, these signaling events lead to attenuated monocyte adhesion onto the endothelial surface (201-204). Silencing AMPKalpha1 has also been reported to decrease the expression of MnSOD, catalase, gamma-glutamylcysteine synthase and thioredoxin, in endothelial cells (205). AMPK also appears to have direct links to NADPH oxidase, a membrane bound enzyme complex which is normally latent in neutrophils and is activated to assemble in the membranes during the respiratory burst. It generates O2.− transferring electrons from NADPH inside the cell across the membrane and coupling these to molecular oxygen to produce O2.−. It has been shown that AICAR or AMP can suppress the production of superoxide anion stimulated by phorbol esters or fMLP (Formyl-Methionyl-Leucyl-Phenylalanine) (206). In neutrophils, AICAR also has the ability to reduce PMA-dependent (phorbol 12-myristate 13-acetate; a potent activator of PKC) H2O2 release and induction of phosphorylation of JNK, p38, MAPK and ERK1/2 (7). The exposure of cultured human endothelial cells (HEC) to high glucose concentrations (10 mM/L) can generate ROS and this can effectively be attenuated by AMPK-activating drugs, such as rosiglitazone (207). AMPK can also influence the cellular redox state via prevention of tyrosine nitration and inhibition of prostacyclin synthase in endothelial cells that have been exposed to high glucose. This has been reported recently by our group when we demonstrated that AICAR could inhibit high glucose-induced nitration and inactivation of prostacyclin synthase in HUVECs, that AMPK activation was necessary for AICAR to reduce oxidative stress, that prostacyclin synthase nitration by AMPK was mediated through an upregulation of UCP-2 (mitochondrial uncoupling protein-2) and that this was dependent on activation of p38 kinase as well (190). These findings from our laboratory are especially of note given that the basic role of uncoupling proteins is to prevent oxidative injury or minimize oxidative stress. In fact, such a mechanism can also been validated in in vivo experiments where AICAR-mediated AMPK activation markedly increases UCP-2 expression and reduction of O2.− and prostacyclin synthase nitration in diabetic wild-type but not in AMPKα-deleted mice (also demonstrated in our report cited above) (190).
VASCULAR SMOOTH MUSCLE DYSFUNCTION
AMPK activation also appears to play a role in vascular pathology that centers on hyperplasia and hypertrophy of vascular tissues as well as angiotensin-II mediated vascular smooth muscle and cardiomyocyte proliferation (208, 209). Moreover, AICAR has been shown to decrease neointimal hyperplasia in the rat femoral artery denudation model linked to ERK1/2 inhibition (in part) (208).
To date, AMPK is the only known regulator that can potentially phosphorylate eNOS on more than one site (Ser117 and Ser633) (7, 48, 210). These are both activating sites in the reductase domain, as well as on Thr495 which is an inhibitory site in the CaM-binding domain (16, 211). A large body of evidence has accumulated that demonstrates that hypoxia, shear stress and thrombin, are all powerful AMPK activators in the endothelial tissues (202, 212-215). In addition, somewhat weaker yet significant stimulation of AMPK is also associated with agents such as VEGF, PPAR agonists, AICAR, metformin and adiponectin in the vascular endothelium (216-219). In 2004, our senior author (M.Z.) and colleagues had reported the biguanide drug, metformin activated AMPK mediated by mitochondrial RNS (reactive nitrogen species) and the PI3K pathway (220). Specifically, this report demonstrated that metformin activated AMPK and increased the phosphorylation of ACC (its downstream effector) at Ser79 in cultured BAEC (bovine aortic endothelial cells), that this was mediated through c-Src and was Pi3K-dependent, was ONOL-dependent, that the peroxynitrile oxidant was sourced to the mitochondrion and that inhibition of mitochondrial complex I activated AMPK. Further, the paper also validated these findings in vivo. Studies from our laboratory have also established that metformin-induced AMPK activation is beneficial to endothelial function via Heat Shock Protein 90 (hsp90) mediated activation of eNOS (221). In this report our group demonstrated that metformin could increase the conversion of arginine into citrulline in BAECs in a dose-dependent fashion, suggesting that NO synthesis was occurring via activation of eNOS. This study also revealed that eNOS activation by metformin was PI3K-dependent, was clearly mediated through the activation of AMPK and that it enhanced the association of hsp90 (an important stress response marker) with eNOS. Recently, our group has also provided evidence that NO itself might act as an endogenous activator of AMPK (222). In this report, we have shown that No activates AMPK in endothelial cells through a Ca2+-dependent mechanism involving CaMKKbeta and that AMPK activation can itself increase NO release through AMPK-dependent phosphorylation of eNOS at Ser1177. These data imply that a positive feedback relationship might exist between eNOS and AMPK activation. However, further investigation of this possibility is warranted.
In terms of the consequences of endothelial activation of AMPK, several aspects need to be borne in mind. Overall, the activation of this molecule by FGF (fibroblast growth factor), adiponectin, hypoxia and VEGF, seems to be critical for angiogenesis (7, 100, 212, 223). For example, dominant negative AMPK (DN-AMPK) is able to suppress both endothelial cell migration in response to VEGF as well as in vitro differentiation into tube-like structures under hypoxic conditions (212). Similarly, increased VEGF expression has been shown in muscle cells treated with AICAR leading to enhanced angiogenic repair in response to ischemic injury in the mouse hind limb. This phenomenon reportedly involves AMPK-dependent activation of p38 MAPK (224). Quite recently, Leick and colleagues have reported that an AMPK/PGC-1alpha-dependent mechanism is likely responsible for exercise-induced VEGF expression in skeletal muscle as well as exercise-training-induced prevention of senescent decline in VEGF protein content (225).
AMPK activation in the endothelium is also linked to endothelial control of vascular smooth muscle function, particularly vasorelaxation by NO which appears to be regulated, at least in part by AMPK activity. Vasorelaxation is a critical arm of vascular tone and tone is a central determinant of blood pressure regulation. Sustained high blood pressure is responsible for a variety of serious disease states such as hypertrophic cardiomyopathy, coronary artery insufficiency and myocardial infarction, hypertensive encephalopathy and cerebrovascular disease, hypertensive retinopathy and hypertensive nephropathy being important examples. It has been established that metformin therapy is beneficial for the cardiovascular system by virtue of its ability to improve vasodilatory function (226, 227). It is possible that these effects of metformin are dependent on eNOS activation as demonstrated by our group and others. Another possibility that merits consideration is the fact that endothelium-dependent vasorelaxation is not exclusively regulated by NO. It is provocative to speculate that AMPK might be linked to vasorelaxation via the generation of epoxyeicosatrienoic acids by the cytochrome P450 epoxygenases (7). In fact LKB1 and AMPK can be activated by stimulation of the constitutive androstane receptor and pregnane X receptor (both of the nuclear receptor superfamily that are thought to be involved in the detoxification of xenobiotics), using Phenobarbital (a classic P450 inducer) (228). In mice that lack hepatic alpha1 and alpha2 isoforms of AMPK, this response is not detectable (229). In keeping with the vasorelaxative role of AMPK, it is now fairly well established that AICAR and metformin can both relax arterial preparations and the evidence for this comes from others as well as our group and senior author (220, 221, 230). Interestingly, there seems to be a species- and vessel-dependent difference in published literature in terms of chemical sensitivity of AMPK activation. For example, unlike rat and mouse aortae, porcine carotid artery smooth muscle is reportedly insensitive to AICAR and metformin, even though this tissue can have AMPK activation by other means, such as by hypoxia and 2-deoxyglucose (which causes a metabolic block in the glycolytic pathway) (231).
AMPK and its link with redox balance as well as NF-kappaB activity provide another putative link between endothelial dysfunction and downregulated AMPK signaling. For instance, hyperglycemia induced endothelial abnormalities can be corrected by AMPK activation, as can the lipotoxicity provoked by substances such as palmitate (232). Indirectly, AMPK activity in skeletal muscle in exercise can also be shown to have beneficial attributes (7).
The role of AMPK activation in anti-atherosclerotic processes in the vasculature is also currently under intense study and several fascinating insights have been revealed. For example, the activation of mTOR by oxidized LDL has been shown to be involved in smooth muscle cell proliferation, and AMPK activation by resveratrol can block the activation of the PI3K/Akt/mTOR/p70S6K pathways with consequent inhibition of DNA synthesis and proliferation of smooth muscle cells (233).
Our group has provided several intriguing lines of empirical information that underscore the beneficial role of AMPK in endothelial cell function. Some have been highlighted above. Others include the novel demonstration that hypoxia-reperfusion via ONOL can activate AMPK in a c-Src mediated, PI3K-dependent manner (234), that the thromboxane receptor (TPr) once stimulated can trigger ROS-mediated LKB1-dependent AMPK activation in vascular smooth muscle resulting in inhibition of cellular protein synthesis (235), that PKCzeta can regulate AMPK activity by increasing phosphorylation at Ser428 of LKB1 (resulting in association of LKB1 with AMPK and consequent phosphorylation of LKB1 itself at Thr172) (236), that the PKCzeta phosphorylation of LKB1 results in nuclear export of the kinase and prompts AMPK activation in endothelial cells (236), and most recently, ROS are required in the process of AMPK activation by statin drugs as well as PKCzeta (237). More recent work (in the process of being prepared for publication) in our laboratory, has revealed a novel mechanism through which H2O2 can mediate AMPK activation independent of changes in the AMP:ATP ratio in endothelial cells. This has been observed in this cell type under nutritional stress and the implicate free radical species appear to be derived from the mitochondria. The most interesting aspect of this recent study is that under nutritive challenge (in our case by utilizing 2-deoxyglucose to induce a biochemical block by preventing glycolytic breakdown at the hexokinase step), AMPK activation led to autophagy rather than cell death. Further, under conditions of hypoxia, endothelial cell death was prevented in an AMPK and ROS dependent manner. Overall, studies from our group reinforce the idea that AMPK activation is acutely sensitive to cellular redox balance and this relationship appears to be independent of its putative function of responding to ATP depletion (or more precisely elevated AMP:ATP) and triggering energy conserving pathways in the cell. AMPK’s response to the redox balance also appears to favor cell survival pathways as evidenced by data from our laboratory as well as from other investigators.
As has been alluded to a previous section, AMPK dysregulation has been linked to obesity and the vascular morbidity observed in the metabolic syndrome and diabetes. There is a buildup of consensus that these vascular consequences may be circumvented, at least theoretically, by pharmaceutical strategies aimed at AMPK activation (238). In clinical use, a number of compounds have modes of action involving AMPK activation and these include popular and effective drugs such as cilostazol, fenofibrate and rosiglitazone (238). Interestingly, polyphenols like Resveratrol also increase phosphorylation of AMPK and have been shown to improve survival in mice who have been fed a high-fat, pro-atherogenic diet (36, 239). In addition, it has been shown that the treatment of type 1 diabetic LDL-receptor deficient mice with a synthetic polyphenols can prevent diabetes-induced decrease in AMPK and ACC phosphorylation and lipid accumulation in the liver and accretion of aortic atherosclerotic lesions (240). Metformin has also been shown to attenuate hyperglycemia and improve insulin sensitivity as well as harboring vasculoprotective effects in many studies including those from our laboratory. For example, it has shown to reduce atherosclerotic lesions in animal models as well as in clinical studies in which the carotid intima-media thickness was measured via ultrasound (241). The endothelial effects of AMPK can largely be mimicked by its commonly used activator, AICAR. For example, in mesenteric vessels isolated from a rat model of type 2 diabetes, the NO and prostacyclin independent relaxation was impaired and endothelium-dependent contraction was enhanced. Metformin and AICAR were both able to correct this imbalance and decrease oxidative stress in this model (242). Overall, from both the bench top and the bedside, the emerging mechanistic picture provides ample encouragement to fine tune a therapeutic approach for AMPK activation in the vasculature that will not only enable better glycemic control but also augment the clinical phenotype of many human diseases by improving vascular endothelial function through this important kinase.
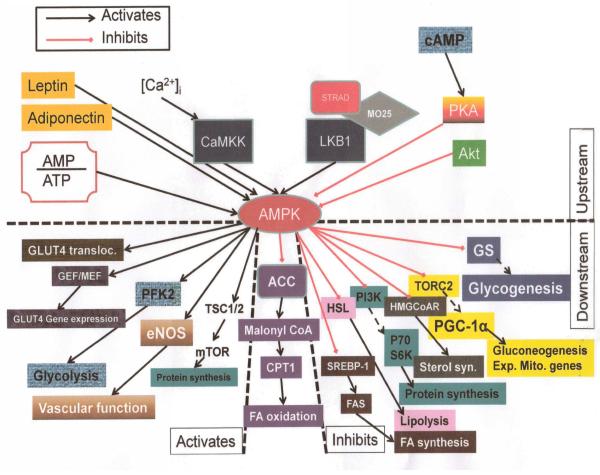
13 SUMMARY AND CONCLUSIONS
It has become evident with continuing research that AMPK plays a pivotal role in the complex dynamics of bioenergetics, in both health and disease. The signaling system that has AMPK as its centerpiece, is fairly complex (Figure 9). A fact that speaks to how far reaching are the effects of activation or inhibition of this kinase. In a physiologic sense, this molecule has layers of influence that it exerts on cellular processes. These include switching between anabolic and catabolic states and altering cellular dynamics by directly influencing genetic controls and protein expression. In conditions where cellular systems are under metabolic strain, AMPK’s ability to quickly correct dynamic processes appears to be an evolutionarily conserved mechanism seen across a wide phylogenetic scale, indeed in all organisms studied thus far. Even when the cellular homeostatic machinery has been overrun by pathological forces, AMPK acquires a central position in buffeting these fluxes, frequently pointing them in a correcting direction and occasionally contributing to the mounting damage that leads to cell death. Some drugs and compounds that have become centerpieces of therapeutics as well as public health dogma, in disorders like diabetes (e.g., metformin), cardiovascular disease (e.g., polyphenols a resveratrol) and infectious diseases (e.g., berberine), appear to deliver their protective/therapeutic effects via modulation of AMPK signaling. However, many important details of underlying mechanisms remain somewhat obscure. There is also the belief among many pioneering and experienced investigators who have studied AMPK for many years (e.g., D.G. Hardie of the University of Dundee), that closer scrutiny of this molecule has also diluted its importance as a regulator, simply because more investigation has revealed many more molecular actors that control bioenergetics. However, at this point AMPK’s dominance as the quintessential metabolic regulator remains relatively unchallenged.
Figure 9.
Integrated AMPK signaling pathways. Top half of schematic represents upstream events. Lower half represents downstream processes. In the lower half, left side are the events that are activated by AMPK while on the right are those that are inhibited by the kinase. In the center of the lower half is the main downstream effector, ACC and the consequence of its inhibition.
ACKNOWLEDGMENTS
The authors sincerely apologize to our colleagues whose original contributions were not cited owing to page limitations. The authors also thank all current and former members of Dr Zou’s laboratory for the work described in this review. Dr. Ming-Hui Zou’s laboratory is supported by funding from the following agencies: National Institutes of Health RO1 (HL110488, HL105157, HL089920, HL080499, HL079584, and HL074399), the American Diabetes Association, the American Heart Association (11SDG5560036), and the Warren Chair in Diabetes Research of the University of Oklahoma Health Sciences Center. Dr. Zou is a recipient of the National Established Investigator Award of the American Heart Association.
REFERENCES
- 1.Alexov EG, Gunner MR. Incorporating protein conformational flexibility into the calculation of pH-dependent protein properties. Biophys J. 1997;72(5):2075–93. doi: 10.1016/S0006-3495(97)78851-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aruksakunwong O, Wittayanarakul K, Sompornpisut P, Sanghiran V, Parasuk V, Hannongbua S. Structural and dynamical properties of different protonated states of mutant HIV-1 protease complexed with the saquinavir inhibitor studied by molecular dynamics simulations. J Mol Graph Model. 2006;25(3):324–32. doi: 10.1016/j.jmgm.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Begg DA, Rebhun LI. pH regulates the polymerization of actin in the sea urchin egg cortex. J Cell Biol. 1979;83(1):241–8. doi: 10.1083/jcb.83.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neuhuber WL. Lung sensors: complex functions require complex structures. Am J Respir Cell Mol Biol. 2003;28(3):265–6. doi: 10.1165/rcmb.F261. [DOI] [PubMed] [Google Scholar]
- 5.Koivusalo M, Kapus A, Grinstein S. Sensors, transducers, and effectors that regulate cell size and shape. J Biol Chem. 2009;284(11):6595–9. doi: 10.1074/jbc.R800049200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouloumie A, Bauersachs J, Linz W, Scholkens BA, Wiemer G, Fleming I, Busse R. Endothelial dysfunction coincides with an enhanced nitric oxide synthase expression and superoxide anion production. Hypertension. 1997;30(4):934–41. doi: 10.1161/01.hyp.30.4.934. [DOI] [PubMed] [Google Scholar]
- 7.Fisslthaler B, Fleming I. Activation and signaling by the AMP-activated protein kinase in endothelial cells. Circ Res. 2009;105(2):114–27. doi: 10.1161/CIRCRESAHA.109.201590. [DOI] [PubMed] [Google Scholar]
- 8.Beg ZH, Allmann DW, Gibson DM. Modulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity with cAMP and wth protein fractions of rat liver cytosol. Biochem Biophys Res Commun. 1973;54(4):1362–9. doi: 10.1016/0006-291x(73)91137-6. [DOI] [PubMed] [Google Scholar]
- 9.Carlson CA, Kim KH. Regulation of hepatic acetyl coenzyme A carboxylase by phosphorylation and dephosphorylation. J Biol Chem. 1973;248(1):378–80. [PubMed] [Google Scholar]
- 10.Munday MR, Campbell DG, Carling D, Hardie DG. Identification by amino acid sequencing of three major regulatory phosphorylation sites on rat acetyl-CoA carboxylase. Eur J Biochem. 1988;175(2):331–8. doi: 10.1111/j.1432-1033.1988.tb14201.x. [DOI] [PubMed] [Google Scholar]
- 11.Carling D, Clarke PR, Zammit VA, Hardie DG. Purification and characterization of the AMP-activated protein kinase. Copurification of acetyl-CoA carboxylase kinase and 3-hydroxy-3-methylglutaryl-CoA reductase kinase activities. Eur J Biochem. 1989;186(1-2):129–36. doi: 10.1111/j.1432-1033.1989.tb15186.x. [DOI] [PubMed] [Google Scholar]
- 12.Steinberg GR, Kemp BE. AMPK in Health and Disease. Physiol Rev. 2009;89(3):1025–78. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 13.Davies SP, Hawley SA, Woods A, Carling D, Haystead TA, Hardie DG. Purification of the AMP-activated protein kinase on ATP-gamma-sepharose and analysis of its subunit structure. Eur J Biochem. 1994;223(2):351–7. doi: 10.1111/j.1432-1033.1994.tb19001.x. [DOI] [PubMed] [Google Scholar]
- 14.Mitchelhill KI, Stapleton D, Gao G, House C, Michell B, Katsis F, Witters LA, Kemp BE. Mammalian AMP-activated protein kinase shares structural and functional homology with the catalytic domain of yeast Snf1 protein kinase. J Biol Chem. 1994;269(4):2361–4. [PubMed] [Google Scholar]
- 15.Stapleton D, Gao G, Michell BJ, Widmer J, Mitchelhill K, Teh T, House CM, Witters LA, Kemp BE. Mammalian 5′-AMP-activated protein kinase non-catalytic subunits are homologs of proteins that interact with yeast Snf1 protein kinase. J Biol Chem. 1994;269(47):29343–6. [PubMed] [Google Scholar]
- 16.Chen Z, Heierhorst J, Mann RJ, Mitchelhill KI, Michell BJ, Witters LA, Lynch GS, Kemp BE, Stapleton D. Expression of the AMP-activated protein kinase beta1 and beta2 subunits in skeletal muscle. FEBS Lett. 1999;460(2):343–8. doi: 10.1016/s0014-5793(99)01371-x. [DOI] [PubMed] [Google Scholar]
- 17.Cheung PC, Salt IP, Davies SP, Hardie DG, Carling D. Characterization of AMP-activated protein kinase gamma-subunit isoforms and their role in AMP binding. Biochem J. 2000;346(Pt 3):659–69. [PMC free article] [PubMed] [Google Scholar]
- 18.Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100(3):328–41. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- 19.Hallows KR, Kobinger GP, Wilson JM, Witters LA, Foskett JK. Physiological modulation of CFTR activity by AMP-activated protein kinase in polarized T84 cells. Am J Physiol Cell Physiol. 2003;284(5):C1297–308. doi: 10.1152/ajpcell.00227.2002. [DOI] [PubMed] [Google Scholar]
- 20.Evans AM, Mustard KJ, Wyatt CN, Peers C, Dipp M, Kumar P, Kinnear NP, Hardie DG. Does AMP-activated protein kinase couple inhibition of mitochondrial oxidative phosphorylation by hypoxia to calcium signaling in O2-sensing cells? J Biol Chem. 2005;280(50):41504–11. doi: 10.1074/jbc.M510040200. [DOI] [PubMed] [Google Scholar]
- 21.Salt IP, Johnson G, Ashcroft SJ, Hardie DG. AMP-activated protein kinase is activated by low glucose in cell lines derived from pancreatic beta cells, and may regulate insulin release. Biochem J. 1998;335(Pt 3):533–9. doi: 10.1042/bj3350533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turnley AM, Stapleton D, Mann RJ, Witters LA, Kemp BE, Bartlett PF. Cellular distribution and developmental expression of AMP-activated protein kinase isoforms in mouse central nervous system. J Neurochem. 1999;72(4):1707–16. doi: 10.1046/j.1471-4159.1999.721707.x. [DOI] [PubMed] [Google Scholar]
- 23.Ai H, Ihlemann J, Hellsten Y, Lauritzen HP, Hardie DG, Galbo H, Ploug T. Effect of fiber type and nutritional state on AICAR- and contraction-stimulated glucose transport in rat muscle. Am J Physiol Endocrinol Metab. 2002;282(6):E1291–300. doi: 10.1152/ajpendo.00167.2001. [DOI] [PubMed] [Google Scholar]
- 24.Polekhina G, Feil SC, Gupta A, O’Donnell P, Stapleton D, Parker MW. Crystallization of the glycogen-binding domain of the AMP-activated protein kinase beta subunit and preliminary X-ray analysis. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2005;61(Pt 1):39–42. doi: 10.1107/S1744309104025059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polekhina G, Gupta A, van Denderen BJ, Feil SC, Kemp BE, Stapleton D, Parker MW. Structural basis for glycogen recognition by AMP-activated protein kinase. Structure. 2005;13(10):1453–62. doi: 10.1016/j.str.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Hudson ER, Pan DA, James J, Lucocq JM, Hawley SA, Green KA, Baba O, Terashima T, Hardie DG. A novel domain in AMP-activated protein kinase causes glycogen storage bodies similar to those seen in hereditary cardiac arrhythmias. Curr Biol. 2003;13(10):861–6. doi: 10.1016/s0960-9822(03)00249-5. [DOI] [PubMed] [Google Scholar]
- 27.Bateman A. The structure of a domain common to archaebacteria and the homocystinuria disease protein. Trends Biochem Sci. 1997;22(1):12–3. doi: 10.1016/s0968-0004(96)30046-7. [DOI] [PubMed] [Google Scholar]
- 28.Scott JW, Hawley SA, Green KA, Anis M, Stewart G, Scullion GA, Norman DG, Hardie DG. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest. 2004;113(2):274–84. doi: 10.1172/JCI19874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229(2):558–65. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- 30.Hardie DG. AMPK and SNF1: Snuffing Out Stress. Cell Metab. 2007;6(5):339–40. doi: 10.1016/j.cmet.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruderman NB, Keller C, Richard AM, Saha AK, Luo Z, Xiang X, Giralt M, Ritov VB, Menshikova EV, Kelley DE, Hidalgo J, Pedersen BK, Kelly M. Interleukin-6 regulation of AMP-activated protein kinase. Potential role in the systemic response to exercise and prevention of the metabolic syndrome. Diabetes. 2006;55(Suppl 2):S48–54. doi: 10.2337/db06-s007. [DOI] [PubMed] [Google Scholar]
- 32.Watt MJ, Dzamko N, Thomas WG, Rose-John S, Ernst M, Carling D, Kemp BE, Febbraio MA, Steinberg GR. CNTF reverses obesity-induced insulin resistance by activating skeletal muscle AMPK. Nat Med. 2006;12(5):541–8. doi: 10.1038/nm1383. [DOI] [PubMed] [Google Scholar]
- 33.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108(8):1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fryer LG, Parbu-Patel A, Carling D. Protein kinase inhibitors block the stimulation of the AMP-activated protein kinase by 5-amino-4-imidazolecarboxamide riboside. FEBS Lett. 2002;531(2):189–92. doi: 10.1016/s0014-5793(02)03501-9. [DOI] [PubMed] [Google Scholar]
- 35.Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, Ye JM, Lee CH, Oh WK, Kim CT, Hohnen-Behrens C, Gosby A, Kraegen EW, James DE, Kim JB. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55(8):2256–64. doi: 10.2337/db06-0006. [DOI] [PubMed] [Google Scholar]
- 36.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–42. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wojtaszewski JF, Nielsen P, Hansen BF, Richter EA, Kiens B. Isoform-specific and exercise intensity-dependent activation of 5′-AMP-activated protein kinase in human skeletal muscle. J Physiol. 2000;528(Pt 1):221–6. doi: 10.1111/j.1469-7793.2000.t01-1-00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warden SM, Richardson C, O’Donnell J, Jr., Stapleton D, Kemp BE, Witters LA. Post-translational modifications of the beta-1 subunit of AMP-activated protein kinase affect enzyme activity and cellular localization. Biochem J. 2001;354(Pt 2):275–83. doi: 10.1042/0264-6021:3540275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hardie DG, Frenguelli BG. A neural protection racket: AMPK and the GABA(B) receptor. Neuron. 2007;53(2):159–62. doi: 10.1016/j.neuron.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Hong SP, Leiper FC, Woods A, Carling D, Carlson M. Activation of yeast Snf1 and mammalian AMP-activated protein kinase by upstream kinases. Proc Natl Acad Sci U S A. 2003;100(15):8839–43. doi: 10.1073/pnas.1533136100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sutherland CM, Hawley SA, McCartney RR, Leech A, Stark MJ, Schmidt MC, Hardie DG. Elm1p is one of three upstream kinases for the Saccharomyces cerevisiae SNF1 complex. Curr Biol. 2003;13(15):1299–305. doi: 10.1016/s0960-9822(03)00459-7. [DOI] [PubMed] [Google Scholar]
- 42.Sakamoto K, Zarrinpashneh E, Budas GR, Pouleur AC, Dutta A, Prescott AR, Vanoverschelde JL, Ashworth A, Jovanovic A, Alessi DR, Bertrand L. Deficiency of LKB1 in heart prevents ischemia-mediated activation of AMPKalpha2 but not AMPKalpha1. Am J Physiol Endocrinol Metab. 2006;290(5):E780–8. doi: 10.1152/ajpendo.00443.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2(1):9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 44.Stahmann N, Woods A, Carling D, Heller R. Thrombin activates AMP-activated protein kinase in endothelial cells via a pathway involving Ca2+/calmodulin-dependent protein kinase kinase beta. Mol Cell Biol. 2006;26(16):5933–45. doi: 10.1128/MCB.00383-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carling D, Hardie DG. The substrate and sequence specificity of the AMP-activated protein kinase. Phosphorylation of glycogen synthase and phosphorylase kinase. Biochim Biophys Acta. 1989;1012(1):81–6. doi: 10.1016/0167-4889(89)90014-1. [DOI] [PubMed] [Google Scholar]
- 46.Clarke PR, Hardie DG. Calmodulin-dependent multiprotein kinase and protein kinase C phosphorylate the same site on HMG-CoA reductase as the AMP-activated protein kinase. FEBS Lett. 1990;269(1):213–7. doi: 10.1016/0014-5793(90)81157-j. [DOI] [PubMed] [Google Scholar]
- 47.Clarke PR, Hardie DG. Regulation of HMG-CoA reductase: identification of the site phosphorylated by the AMP-activated protein kinase in vitro and in intact rat liver. EMBO J. 1990;9(8):2439–46. doi: 10.1002/j.1460-2075.1990.tb07420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999;443(3):285–9. doi: 10.1016/s0014-5793(98)01705-0. [DOI] [PubMed] [Google Scholar]
- 49.Jakobsen SN, Hardie DG, Morrice N, Tornqvist HE. 5′-AMP-activated protein kinase phosphorylates IRS-1 on Ser-789 in mouse C2C12 myotubes in response to 5-aminoimidazole-4-carboxamide riboside. J Biol Chem. 2001;276(50):46912–6. doi: 10.1074/jbc.C100483200. [DOI] [PubMed] [Google Scholar]
- 50.Jiang XP, Yang DC, Elliott RL, Head JF. Down-regulation of expression of interleukin-6 and its receptor results in growth inhibition of MCF-7 breast cancer cells. Anticancer Res. 31(9):2899–906. (Year) [PubMed] [Google Scholar]
- 51.Hong YH, Varanasi US, Yang W, Leff T. AMP-activated protein kinase regulates HNF4alpha transcriptional activity by inhibiting dimer formation and decreasing protein stability. J Biol Chem. 2003;278(30):27495–501. doi: 10.1074/jbc.M304112200. [DOI] [PubMed] [Google Scholar]
- 52.Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, Takemori H, Montminy M. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437(7062):1109–11. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- 53.Weekes J, Ball KL, Caudwell FB, Hardie DG. Specificity determinants for the AMP-activated protein kinase and its plant homologue analysed using synthetic peptides. FEBS Lett. 1993;334(3):335–9. doi: 10.1016/0014-5793(93)80706-z. [DOI] [PubMed] [Google Scholar]
- 54.Dale S, Wilson WA, Edelman AM, Hardie DG. Similar substrate recognition motifs for mammalian AMP-activated protein kinase, higher plant HMG-CoA reductase kinase-A, yeast SNF1, and mammalian calmodulin-dependent protein kinase I. FEBS Lett. 1995;361(2-3):191–5. doi: 10.1016/0014-5793(95)00172-6. [DOI] [PubMed] [Google Scholar]
- 55.Geraghty KM, Chen S, Harthill JE, Ibrahim AF, Toth R, Morrice NA, Vandermoere F, Moorhead GB, Hardie DG, MacKintosh C. Regulation of multisite phosphorylation and 14-3-3 binding of AS160 in response to IGF-1, EGF, PMA and AICAR. Biochem J. 2007;407(2):231–41. doi: 10.1042/BJ20070649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng D, MacLean PS, Pohnert SC, Knight JB, Olson AL, Winder WW, Dohm GL. Regulation of muscle GLUT-4 transcription by AMP-activated protein kinase. J Appl Physiol. 2001;91(3):1073–83. doi: 10.1152/jappl.2001.91.3.1073. [DOI] [PubMed] [Google Scholar]
- 57.Lin SS, Manchester JK, Gordon JI. Enhanced gluconeogenesis and increased energy storage as hallmarks of aging in Saccharomyces cerevisiae. J Biol Chem. 2001;276(38):36000–7. doi: 10.1074/jbc.M103509200. [DOI] [PubMed] [Google Scholar]
- 58.Young ME, Radda GK, Leighton B. Activation of glycogen phosphorylase and glycogenolysis in rat skeletal muscle by AICAR--an activator of AMP-activated protein kinase. FEBS Lett. 1996;382(1-2):43–7. doi: 10.1016/0014-5793(96)00129-9. [DOI] [PubMed] [Google Scholar]
- 59.Jorgensen SB, Nielsen JN, Birk JB, Olsen GS, Viollet B, Andreelli F, Schjerling P, Vaulont S, Hardie DG, Hansen BF, Richter EA, Wojtaszewski JF. The alpha2-5′AMP-activated protein kinase is a site 2 glycogen synthase kinase in skeletal muscle and is responsive to glucose loading. Diabetes. 2004;53(12):3074–81. doi: 10.2337/diabetes.53.12.3074. [DOI] [PubMed] [Google Scholar]
- 60.Bergeron R, Ren JM, Cadman KS, Moore IK, Perret P, Pypaert M, Young LH, Semenkovich CF, Shulman GI. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am J Physiol Endocrinol Metab. 2001;281(6):E1340–6. doi: 10.1152/ajpendo.2001.281.6.E1340. [DOI] [PubMed] [Google Scholar]
- 61.Andreelli F, Foretz M, Knauf C, Cani PD, Perrin C, Iglesias MA, Pillot B, Bado A, Tronche F, Mithieux G, Vaulont S, Burcelin R, Viollet B. Liver adenosine monophosphate-activated kinase-alpha2 catalytic subunit is a key target for the control of hepatic glucose production by adiponectin and leptin but not insulin. Endocrinology. 2006;147(5):2432–41. doi: 10.1210/en.2005-0898. [DOI] [PubMed] [Google Scholar]
- 62.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8(11):1288–95. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 63.da Silva Xavier G, Leclerc I, Varadi A, Tsuboi T, Moule SK, Rutter GA. Role for AMP-activated protein kinase in glucose-stimulated insulin secretion and preproinsulin gene expression. Biochem J. 2003;371(Pt 3):761–74. doi: 10.1042/BJ20021812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lochhead PA, Salt IP, Walker KS, Hardie DG, Sutherland C. 5-aminoimidazole-4-carboxamide riboside mimics the effects of insulin on the expression of the 2 key gluconeogenic genes PEPCK and glucose-6-phosphatase. Diabetes. 2000;49(6):896–903. doi: 10.2337/diabetes.49.6.896. [DOI] [PubMed] [Google Scholar]
- 65.Woods A, Azzout-Marniche D, Foretz M, Stein SC, Lemarchand P, Ferre P, Foufelle F, Carling D. Characterization of the role of AMP-activated protein kinase in the regulation of glucose-activated gene expression using constitutively active and dominant negative forms of the kinase. Mol Cell Biol. 2000;20(18):6704–11. doi: 10.1128/mcb.20.18.6704-6711.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310(5754):1642–6. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kiens B. Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol Rev. 2006;86(1):205–43. doi: 10.1152/physrev.00023.2004. [DOI] [PubMed] [Google Scholar]
- 68.Voet JG. DV: Biochemistry. Wiley; 2004. [Google Scholar]
- 69.Davies SP, Sim AT, Hardie DG. Location and function of three sites phosphorylated on rat acetyl-CoA carboxylase by the AMP-activated protein kinase. Eur J Biochem. 1990;187(1):183–90. doi: 10.1111/j.1432-1033.1990.tb15293.x. [DOI] [PubMed] [Google Scholar]
- 70.Ha J, Daniel S, Broyles SS, Kim KH. Critical phosphorylation sites for acetyl-CoA carboxylase activity. J Biol Chem. 1994;269(35):22162–8. [PubMed] [Google Scholar]
- 71.Baron A, Migita T, Tang D, Loda M. Fatty acid synthase: a metabolic oncogene in prostate cancer? J Cell Biochem. 2004;91(1):47–53. doi: 10.1002/jcb.10708. [DOI] [PubMed] [Google Scholar]
- 72.Foretz M, Carling D, Guichard C, Ferre P, Foufelle F. AMP-activated protein kinase inhibits the glucose-activated expression of fatty acid synthase gene in rat hepatocytes. J Biol Chem. 1998;273(24):14767–71. doi: 10.1074/jbc.273.24.14767. [DOI] [PubMed] [Google Scholar]
- 73.Foretz M, Ancellin N, Andreelli F, Saintillan Y, Grondin P, Kahn A, Thorens B, Vaulont S, Viollet B. Short-term overexpression of a constitutively active form of AMP-activated protein kinase in the liver leads to mild hypoglycemia and fatty liver. Diabetes. 2005;54(5):1331–9. doi: 10.2337/diabetes.54.5.1331. [DOI] [PubMed] [Google Scholar]
- 74.An Z, Wang H, Song P, Zhang M, Geng X, Zou MH. Nicotine-induced activation of AMP-activated protein kinase inhibits fatty acid synthase in 3T3L1 adipocytes: a role for oxidant stress. J Biol Chem. 2007;282(37):26793–801. doi: 10.1074/jbc.M703701200. [DOI] [PubMed] [Google Scholar]
- 75.Lewin TM, Schwerbrock NM, Lee DP, Coleman RA. Identification of a new glycerol-3-phosphate acyltransferase isoenzyme, mtGPAT2, in mitochondria. J Biol Chem. 2004;279(14):13488–95. doi: 10.1074/jbc.M314032200. [DOI] [PubMed] [Google Scholar]
- 76.Muoio DM, Seefeld K, Witters LA, Coleman RA. AMP-activated kinase reciprocally regulates triacylglycerol synthesis and fatty acid oxidation in liver and muscle: evidence that sn-glycerol-3-phosphate acyltransferase is a novel target. Biochem J. 1999;338(Pt 3):783–91. [PMC free article] [PubMed] [Google Scholar]
- 77.Watt MJ, Holmes AG, Steinberg GR, Mesa JL, Kemp BE, Febbraio MA. Reduced plasma FFA availability increases net triacylglycerol degradation, but not GPAT or HSL activity, in human skeletal muscle. Am J Physiol Endocrinol Metab. 2004;287(1):E120–7. doi: 10.1152/ajpendo.00542.2003. [DOI] [PubMed] [Google Scholar]
- 78.Witters LA, Gao G, Kemp BE, Quistorff B. Hepatic 5′-AMP-activated protein kinase: zonal distribution and relationship to acetyl-CoA carboxylase activity in varying nutritional states. Arch Biochem Biophys. 1994;308(2):413–9. doi: 10.1006/abbi.1994.1058. [DOI] [PubMed] [Google Scholar]
- 79.Assifi MM, Suchankova G, Constant S, Prentki M, Saha AK, Ruderman NB. AMP-activated protein kinase and coordination of hepatic fatty acid metabolism of starved/carbohydrate-refed rats. Am J Physiol Endocrinol Metab. 2005;289(5):E794–800. doi: 10.1152/ajpendo.00144.2005. [DOI] [PubMed] [Google Scholar]
- 80.Daval M, Diot-Dupuy F, Bazin R, Hainault I, Viollet B, Vaulont S, Hajduch E, Ferre P, Foufelle F. Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. J Biol Chem. 2005;280(26):25250–7. doi: 10.1074/jbc.M414222200. [DOI] [PubMed] [Google Scholar]
- 81.Fisher JS, Ju JS, Oppelt PJ, Smith JL, Suzuki A, Esumi H. Muscle contractions, AICAR, and insulin cause phosphorylation of an AMPK-related kinase. Am J Physiol Endocrinol Metab. 2005;289(6):E986–92. doi: 10.1152/ajpendo.00335.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Smith AC, Bruce CR, Dyck DJ. AMP kinase activation with AICAR simultaneously increases fatty acid and glucose oxidation in resting rat soleus muscle. J Physiol. 2005;565(Pt 2):537–46. doi: 10.1113/jphysiol.2004.081679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Smith JL, Patil PB, Fisher JS. AICAR and hyperosmotic stress increase insulin-stimulated glucose transport. J Appl Physiol. 2005;99(3):877–83. doi: 10.1152/japplphysiol.01297.2004. [DOI] [PubMed] [Google Scholar]
- 84.Clark H, Carling D, Saggerson D. Covalent activation of heart AMP-activated protein kinase in response to physiological concentrations of long-chain fatty acids. Eur J Biochem. 2004;271(11):2215–24. doi: 10.1111/j.1432-1033.2004.04151.x. [DOI] [PubMed] [Google Scholar]
- 85.Suchankova G, Tekle M, Saha AK, Ruderman NB, Clarke SD, Gettys TW. Dietary polyunsaturated fatty acids enhance hepatic AMP-activated protein kinase activity in rats. Biochem Biophys Res Commun. 2005;326(4):851–8. doi: 10.1016/j.bbrc.2004.11.114. [DOI] [PubMed] [Google Scholar]
- 86.Kelley DE, Simoneau JA. Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus. J Clin Invest. 1994;94(6):2349–56. doi: 10.1172/JCI117600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Muse ED, Obici S, Bhanot S, Monia BP, McKay RA, Rajala MW, Scherer PE, Rossetti L. Role of resistin in diet-induced hepatic insulin resistance. J Clin Invest. 2004;114(2):232–9. doi: 10.1172/JCI21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wilkes JJ, Nguyen MT, Bandyopadhyay GK, Nelson E, Olefsky JM. Topiramate treatment causes skeletal muscle insulin sensitization and increased Acrp30 secretion in high-fat-fed male Wistar rats. Am J Physiol Endocrinol Metab. 2005;289(6):E1015–22. doi: 10.1152/ajpendo.00169.2005. [DOI] [PubMed] [Google Scholar]
- 89.Lee WJ, Lee IK, Kim HS, Kim YM, Koh EH, Won JC, Han SM, Kim MS, Jo I, Oh GT, Park IS, Youn JH, Park SW, Lee KU, Park JY. Alpha-lipoic acid prevents endothelial dysfunction in obese rats via activation of AMP-activated protein kinase. Arterioscler Thromb Vasc Biol. 2005;25(12):2488–94. doi: 10.1161/01.ATV.0000190667.33224.4c. [DOI] [PubMed] [Google Scholar]
- 90.Lessard SJ, Chen ZP, Watt MJ, Hashem M, Reid JJ, Febbraio MA, Kemp BE, Hawley JA. Chronic rosiglitazone treatment restores AMPKalpha2 activity in insulin-resistant rat skeletal muscle. Am J Physiol Endocrinol Metab. 2006;290(2):E251–7. doi: 10.1152/ajpendo.00096.2005. [DOI] [PubMed] [Google Scholar]
- 91.Wu Y, Song P, Xu J, Zhang M, Zou MH. Activation of protein phosphatase 2A by palmitate inhibits AMP-activated protein kinase. J Biol Chem. 2007;282(13):9777–88. doi: 10.1074/jbc.M608310200. [DOI] [PubMed] [Google Scholar]
- 92.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350(7):664–71. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol. 2000;88(6):2219–26. doi: 10.1152/jappl.2000.88.6.2219. [DOI] [PubMed] [Google Scholar]
- 94.McGee SL, Hargreaves M. AMPK and transcriptional regulation. Front Biosci. 2008;13:3022–33. doi: 10.2741/2907. [DOI] [PubMed] [Google Scholar]
- 95.Iglesias MA, Furler SM, Cooney GJ, Kraegen EW, Ye JM. AMP-activated protein kinase activation by AICAR increases both muscle fatty acid and glucose uptake in white muscle of insulin-resistant rats in vivo. Diabetes. 2004;53(7):1649–54. doi: 10.2337/diabetes.53.7.1649. [DOI] [PubMed] [Google Scholar]
- 96.Watt MJ, Steinberg GR, Chan S, Garnham A, Kemp BE, Febbraio MA. Beta-adrenergic stimulation of skeletal muscle HSL can be overridden by AMPK signaling. FASEB J. 2004;18(12):1445–6. doi: 10.1096/fj.03-1067fje. [DOI] [PubMed] [Google Scholar]
- 97.Kukidome D, Nishikawa T, Sonoda K, Imoto K, Fujisawa K, Yano M, Motoshima H, Taguchi T, Matsumura T, Araki E. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes. 2006;55(1):120–7. [PubMed] [Google Scholar]
- 98.Beg ZH, Stonik JA, Brewer HB., Jr. 3-Hydroxy-3-methylglutaryl coenzyme A reductase: regulation of enzymatic activity by phosphorylation and dephosphorylation. Proc Natl Acad Sci U S A. 1978;75(8):3678–82. doi: 10.1073/pnas.75.8.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Beg ZH, Stonik JA, Brewer HB., Jr. Solubilization of 3-hydroxy-3-methylglutaryl coenzyme A reductase from rat and chicken liver microsomes. Anal Biochem. 1978;86(2):531–5. doi: 10.1016/0003-2697(78)90779-0. [DOI] [PubMed] [Google Scholar]
- 100.Ouchi N, Kihara S, Arita Y, Nishida M, Matsuyama A, Okamoto Y, Ishigami M, Kuriyama H, Kishida K, Nishizawa H, Hotta K, Muraguchi M, Ohmoto Y, Yamashita S, Funahashi T, Matsuzawa Y. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103(8):1057–63. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- 101.Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang Cc C, Itani SI, Lodish HF, Ruderman NB. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci U S A. 2002;99(25):16309–13. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Browne GJ, Finn SG, Proud CG. Stimulation of the AMP-activated protein kinase leads to activation of eukaryotic elongation factor 2 kinase and to its phosphorylation at a novel site, serine 398. J Biol Chem. 2004;279(13):12220–31. doi: 10.1074/jbc.M309773200. [DOI] [PubMed] [Google Scholar]
- 103.Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17(15):1829–34. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115(5):577–90. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 105.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30(2):214–26. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mizukoshi E, Suzuki M, Misono T, Loupatov A, Munekata E, Kaul SC, Wadhwa R, Imamura T. Cell-cycle dependent tyrosine phosphorylation on mortalin regulates its interaction with fibroblast growth factor-1. Biochem Biophys Res Commun. 2001;280(4):1203–9. doi: 10.1006/bbrc.2001.4225. [DOI] [PubMed] [Google Scholar]
- 107.Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18(3):283–93. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 108.Rattan R, Giri S, Singh AK, Singh I. 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside inhibits cancer cell proliferation in vitro and in vivo via AMP-activated protein kinase. J Biol Chem. 2005;280(47):39582–93. doi: 10.1074/jbc.M507443200. [DOI] [PubMed] [Google Scholar]
- 109.Wang W, Fan J, Yang X, Furer-Galban S, Lopez de Silanes I, von Kobbe C, Guo J, Georas SN, Foufelle F, Hardie DG, Carling D, Gorospe M. AMP-activated kinase regulates cytoplasmic HuR. Mol Cell Biol. 2002;22(10):3425–36. doi: 10.1128/MCB.22.10.3425-3436.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hallows KR, McCane JE, Kemp BE, Witters LA, Foskett JK. Regulation of channel gating by AMP-activated protein kinase modulates cystic fibrosis transmembrane conductance regulator activity in lung submucosal cells. J Biol Chem. 2003;278(2):998–1004. doi: 10.1074/jbc.M210621200. [DOI] [PubMed] [Google Scholar]
- 111.Carattino MD, Edinger RS, Grieser HJ, Wise R, Neumann D, Schlattner U, Johnson JP, Kleyman TR, Hallows KR. Epithelial sodium channel inhibition by AMP-activated protein kinase in oocytes and polarized renal epithelial cells. J Biol Chem. 2005;280(18):17608–16. doi: 10.1074/jbc.M501770200. [DOI] [PubMed] [Google Scholar]
- 112.Fraser SA, Gimenez I, Cook N, Jennings I, Katerelos M, Katsis F, Levidiotis V, Kemp BE, Power DA. Regulation of the renal-specific Na+-K+-2Cl− co-transporter NKCC2 by AMP-activated protein kinase (AMPK) Biochem J. 2007;405(1):85–93. doi: 10.1042/BJ20061850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Klein H, Garneau L, Trinh NT, Prive A, Dionne F, Goupil E, Thuringer D, Parent L, Brochiero E, Sauve R. Inhibition of the KCa3.1 channels by AMP-activated protein kinase in human airway epithelial cells. Am J Physiol Cell Physiol. 2009;296(2):C285–95. doi: 10.1152/ajpcell.00418.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Steinberg GR, Watt MJ, Ernst M, Birnbaum MJ, Kemp BE, Jorgensen SB. Ciliary neurotrophic factor stimulates muscle glucose uptake by a PI3-kinase-dependent pathway that is impaired with obesity. Diabetes. 2009;58(4):829–39. doi: 10.2337/db08-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fediuc S, Gaidhu MP, Ceddia RB. Regulation of AMP-activated protein kinase and acetyl-CoA carboxylase phosphorylation by palmitate in skeletal muscle cells. J Lipid Res. 2006;47(2):412–20. doi: 10.1194/jlr.M500438-JLR200. [DOI] [PubMed] [Google Scholar]
- 116.Dobrzyn A, Dobrzyn P, Lee SH, Miyazaki M, Cohen P, Asilmaz E, Hardie DG, Friedman JM, Ntambi JM. Stearoyl-CoA desaturase-1 deficiency reduces ceramide synthesis by downregulating serine palmitoyltransferase and increasing beta-oxidation in skeletal muscle. Am J Physiol Endocrinol Metab. 2005;288(3):E599–607. doi: 10.1152/ajpendo.00439.2004. [DOI] [PubMed] [Google Scholar]
- 117.Maeda K, Cao H, Kono K, Gorgun CZ, Furuhashi M, Uysal KT, Cao Q, Atsumi G, Malone H, Krishnan B, Minokoshi Y, Kahn BB, Parker RA, Hotamisligil GS. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab. 2005;1(2):107–19. doi: 10.1016/j.cmet.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 118.Cao H, Maeda K, Gorgun CZ, Kim HJ, Park SY, Shulman GI, Kim JK, Hotamisligil GS. Regulation of metabolic responses by adipocyte/macrophage Fatty Acid-binding proteins in leptin-deficient mice. Diabetes. 2006;55(7):1915–22. doi: 10.2337/db05-1496. [DOI] [PubMed] [Google Scholar]
- 119.Gleason CE, Lu D, Witters LA, Newgard CB, Birnbaum MJ. The role of AMPK and mTOR in nutrient sensing in pancreatic beta-cells. J Biol Chem. 2007;282(14):10341–51. doi: 10.1074/jbc.M610631200. [DOI] [PubMed] [Google Scholar]
- 120.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428(6982):569–74. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 121.Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem. 2004;279(13):12005–8. doi: 10.1074/jbc.C300557200. [DOI] [PubMed] [Google Scholar]
- 122.Hutber CA, Hardie DG, Winder WW. Electrical stimulation inactivates muscle acetyl-CoA carboxylase and increases AMP-activated protein kinase. Am J Physiol. 1997;272(2 Pt 1):E262–6. doi: 10.1152/ajpendo.1997.272.2.E262. [DOI] [PubMed] [Google Scholar]
- 123.Vavvas D, Apazidis A, Saha AK, Gamble J, Patel A, Kemp BE, Witters LA, Ruderman NB. Contraction-induced changes in acetyl-CoA carboxylase and 5′-AMP-activated kinase in skeletal muscle. J Biol Chem. 1997;272(20):13255–61. doi: 10.1074/jbc.272.20.13255. [DOI] [PubMed] [Google Scholar]
- 124.Sakamoto K, McCarthy A, Smith D, Green KA, Grahame Hardie D, Ashworth A, Alessi DR. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J. 2005;24(10):1810–20. doi: 10.1038/sj.emboj.7600667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Koh HJ, Arnolds DE, Fujii N, Tran TT, Rogers MJ, Jessen N, Li Y, Liew CW, Ho RC, Hirshman MF, Kulkarni RN, Kahn CR, Goodyear LJ. Skeletal muscle-selective knockout of LKB1 increases insulin sensitivity, improves glucose homeostasis, and decreases TRB3. Mol Cell Biol. 2006;26(22):8217–27. doi: 10.1128/MCB.00979-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Coven DL, Hu X, Cong L, Bergeron R, Shulman GI, Hardie DG, Young LH. Physiological role of AMP-activated protein kinase in the heart: graded activation during exercise. Am J Physiol Endocrinol Metab. 2003;285(3):E629–36. doi: 10.1152/ajpendo.00171.2003. [DOI] [PubMed] [Google Scholar]
- 127.Jorgensen SB, Viollet B, Andreelli F, Frosig C, Birk JB, Schjerling P, Vaulont S, Richter EA, Wojtaszewski JF. Knockout of the alpha2 but not alpha1 5′-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranosidebut not contraction-induced glucose uptake in skeletal muscle. J Biol Chem. 2004;279(2):1070–9. doi: 10.1074/jbc.M306205200. [DOI] [PubMed] [Google Scholar]
- 128.Fujii N, Hirshman MF, Kane EM, Ho RC, Peter LE, Seifert MM, Goodyear LJ. AMP-activated protein kinase alpha2 activity is not essential for contraction- and hyperosmolarity-induced glucose transport in skeletal muscle. J Biol Chem. 2005;280(47):39033–41. doi: 10.1074/jbc.M504208200. [DOI] [PubMed] [Google Scholar]
- 129.Jensen TE, Rose AJ, Jorgensen SB, Brandt N, Schjerling P, Wojtaszewski JF, Richter EA. Possible CaMKK-dependent regulation of AMPK phosphorylation and glucose uptake at the onset of mild tetanic skeletal muscle contraction. Am J Physiol Endocrinol Metab. 2007;292(5):E1308–17. doi: 10.1152/ajpendo.00456.2006. [DOI] [PubMed] [Google Scholar]
- 130.Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol. 1997;273(6 Pt 1):E1107–12. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]
- 131.Kaushik VK, Young ME, Dean DJ, Kurowski TG, Saha AK, Ruderman NB. Regulation of fatty acid oxidation and glucose metabolism in rat soleus muscle: effects of AICAR. Am J Physiol Endocrinol Metab. 2001;281(2):E335–40. doi: 10.1152/ajpendo.2001.281.2.E335. [DOI] [PubMed] [Google Scholar]
- 132.Thomson DM, Brown JD, Fillmore N, Condon BM, Kim HJ, Barrow JR, Winder WW. LKB1 and the regulation of malonyl-CoA and fatty acid oxidation in muscle. Am J Physiol Endocrinol Metab. 2007;293(6):E1572–9. doi: 10.1152/ajpendo.00371.2007. [DOI] [PubMed] [Google Scholar]
- 133.Raney MA, Turcotte LP. Regulation of contraction-induced FA uptake and oxidation by AMPK and ERK1/2 is intensity dependent in rodent muscle. Am J Physiol Endocrinol Metab. 2006;291(6):E1220–7. doi: 10.1152/ajpendo.00155.2006. [DOI] [PubMed] [Google Scholar]
- 134.Shearer J, Fueger PT, Vorndick B, Bracy DP, Rottman JN, Clanton JA, Wasserman DH. AMP kinase-induced skeletal muscle glucose but not long-chain fatty acid uptake is dependent on nitric oxide. Diabetes. 2004;53(6):1429–35. doi: 10.2337/diabetes.53.6.1429. [DOI] [PubMed] [Google Scholar]
- 135.Chen ZP, McConell GK, Michell BJ, Snow RJ, Canny BJ, Kemp BE. AMPK signaling in contracting human skeletal muscle: acetyl-CoA carboxylase and NO synthase phosphorylation. Am J Physiol Endocrinol Metab. 2000;279(5):E1202–6. doi: 10.1152/ajpendo.2000.279.5.E1202. [DOI] [PubMed] [Google Scholar]
- 136.Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415(6869):339–43. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 137.Yu X, McCorkle S, Wang M, Lee Y, Li J, Saha AK, Unger RH, Ruderman NB. Leptinomimetic effects of the AMP kinase activator AICAR in leptin-resistant rats: prevention of diabetes and ectopic lipid deposition. Diabetologia. 2004;47(11):2012–21. doi: 10.1007/s00125-004-1570-9. [DOI] [PubMed] [Google Scholar]
- 138.Brabant G, Muller G, Horn R, Anderwald C, Roden M, Nave H. Hepatic leptin signaling in obesity. FASEB J. 2005;19(8):1048–50. doi: 10.1096/fj.04-2846fje. [DOI] [PubMed] [Google Scholar]
- 139.Atkinson LL, Fischer MA, Lopaschuk GD. Leptin activates cardiac fatty acid oxidation independent of changes in the AMP-activated protein kinase-acetyl-CoA carboxylase-malonyl-CoA axis. J Biol Chem. 2002;277(33):29424–30. doi: 10.1074/jbc.M203813200. [DOI] [PubMed] [Google Scholar]
- 140.Lee Y, Naseem RH, Duplomb L, Park BH, Garry DJ, Richardson JA, Schaffer JE, Unger RH. Hyperleptinemia prevents lipotoxic cardiomyopathy in acyl CoA synthase transgenic mice. Proc Natl Acad Sci U S A. 2004;101(37):13624–9. doi: 10.1073/pnas.0405499101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lim CT, Kola B, Korbonits M. AMPK as a mediator of hormonal signalling. J Mol Endocrinol. 44(2):87–97. doi: 10.1677/JME-09-0063. [DOI] [PubMed] [Google Scholar]
- 142.Huypens P, Moens K, Heimberg H, Ling Z, Pipeleers D, Van de Casteele M. Adiponectin-mediated stimulation of AMP-activated protein kinase (AMPK) in pancreatic beta cells. Life Sci. 2005;77(11):1273–82. doi: 10.1016/j.lfs.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 143.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11(10):1096–103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liao Y, Takashima S, Maeda N, Ouchi N, Komamura K, Shimomura I, Hori M, Matsuzawa Y, Funahashi T, Kitakaze M. Exacerbation of heart failure in adiponectin-deficient mice due to impaired regulation of AMPK and glucose metabolism. Cardiovasc Res. 2005;67(4):705–13. doi: 10.1016/j.cardiores.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 145.Vazquez MJ, Gonzalez CR, Varela L, Lage R, Tovar S, Sangiao-Alvarellos S, Williams LM, Vidal-Puig A, Lopez M, Dieguez C. Central resistin regulates hypothalamic and peripheral lipid metabolism in a nutritional-dependent fashion. Endocrinology. 2008;149(9):4534–43. doi: 10.1210/en.2007-1708. [DOI] [PubMed] [Google Scholar]
- 146.Christ-Crain M, Kola B, Lolli F, Fekete C, Seboek D, Wittmann G, Feltrin D, Igreja SC, Ajodha S, Harvey-White J, Kunos G, Muller B, Pralong F, Aubert G, Arnaldi G, Giacchetti G, Boscaro M, Grossman AB, Korbonits M. AMP-activated protein kinase mediates glucocorticoid-induced metabolic changes: a novel mechanism in Cushing’s syndrome. FASEB J. 2008;22(6):1672–83. doi: 10.1096/fj.07-094144. [DOI] [PubMed] [Google Scholar]
- 147.Kola B. Role of AMP-activated protein kinase in the control of appetite. J Neuroendocrinol. 2008;20(7):942–51. doi: 10.1111/j.1365-2826.2008.01745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kola B, Christ-Crain M, Lolli F, Arnaldi G, Giacchetti G, Boscaro M, Grossman AB, Korbonits M. Changes in adenosine 5′-monophosphate-activated protein kinase as a mechanism of visceral obesity in Cushing’s syndrome. J Clin Endocrinol Metab. 2008;93(12):4969–73. doi: 10.1210/jc.2008-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Namkoong C, Kim MS, Jang PG, Han SM, Park HS, Koh EH, Lee WJ, Kim JY, Park IS, Park JY, Lee KU. Enhanced hypothalamic AMP-activated protein kinase activity contributes to hyperphagia in diabetic rats. Diabetes. 2005;54(1):63–8. doi: 10.2337/diabetes.54.1.63. [DOI] [PubMed] [Google Scholar]
- 150.Horman S, Vertommen D, Heath R, Neumann D, Mouton V, Woods A, Schlattner U, Wallimann T, Carling D, Hue L, Rider MH. Insulin antagonizes ischemia-induced Thr172 phosphorylation of AMP-activated protein kinase alpha-subunits in heart via hierarchical phosphorylation of Ser485/491. J Biol Chem. 2006;281(9):5335–40. doi: 10.1074/jbc.M506850200. [DOI] [PubMed] [Google Scholar]
- 151.Barnes K, Ingram JC, Porras OH, Barros LF, Hudson ER, Fryer LG, Foufelle F, Carling D, Hardie DG, Baldwin SA. Activation of GLUT1 by metabolic and osmotic stress: potential involvement of AMP-activated protein kinase (AMPK) J Cell Sci. 2002;115(Pt 11):2433–42. doi: 10.1242/jcs.115.11.2433. [DOI] [PubMed] [Google Scholar]
- 152.Fisher JS, Gao J, Han DH, Holloszy JO, Nolte LA. Activation of AMP kinase enhances sensitivity of muscle glucose transport to insulin. Am J Physiol Endocrinol Metab. 2002;282(1):E18–23. doi: 10.1152/ajpendo.2002.282.1.E18. [DOI] [PubMed] [Google Scholar]
- 153.Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, Barnett J, Leslie NR, Cheng S, Shepherd PR, Gout I, Downes CP, Lamb RF. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166(2):213–23. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Barness LA, Opitz JM, Gilbert-Barness E. Obesity: genetic, molecular, and environmental aspects. Am J Med Genet A. 2007;143A(24):3016–34. doi: 10.1002/ajmg.a.32035. doi:10.1002/ajmg.a.32035. [DOI] [PubMed] [Google Scholar]
- 155.Buhl ES, Jessen N, Pold R, Ledet T, Flyvbjerg A, Pedersen SB, Pedersen O, Schmitz O, Lund S. Long-term AICAR administration reduces metabolic disturbances and lowers blood pressure in rats displaying features of the insulin resistance syndrome. Diabetes. 2002;51(7):2199–206. doi: 10.2337/diabetes.51.7.2199. [DOI] [PubMed] [Google Scholar]
- 156.Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science. 2001;291(5513):2613–6. doi: 10.1126/science.1056843. [DOI] [PubMed] [Google Scholar]
- 157.Hammond LE, Gallagher PA, Wang S, Hiller S, Kluckman KD, Posey-Marcos EL, Maeda N, Coleman RA. Mitochondrial glycerol-3-phosphate acyltransferase-deficient mice have reduced weight and liver triacylglycerol content and altered glycerolipid fatty acid composition. Mol Cell Biol. 2002;22(23):8204–14. doi: 10.1128/MCB.22.23.8204-8214.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wu X, Motoshima H, Mahadev K, Stalker TJ, Scalia R, Goldstein BJ. Involvement of AMP-activated protein kinase in glucose uptake stimulated by the globular domain of adiponectin in primary rat adipocytes. Diabetes. 2003;52(6):1355–63. doi: 10.2337/diabetes.52.6.1355. [DOI] [PubMed] [Google Scholar]
- 159.Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, Gygi SP, Brunet A. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol. 2007;17(19):1646–56. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ingram DK, Anson RM, de Cabo R, Mamczarz J, Zhu M, Mattison J, Lane MA, Roth GS. Development of calorie restriction mimetics as a prolongevity strategy. Ann N Y Acad Sci. 2004;1019:412–23. doi: 10.1196/annals.1297.074. [DOI] [PubMed] [Google Scholar]
- 161.Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, Kovalenko IG, Poroshina TE, Semenchenko AV, Provinciali M, Re F, Franceschi C. Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Exp Gerontol. 2005;40(8-9):685–93. doi: 10.1016/j.exger.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 162.Anisimov VN, Egormin PA, Bershtein LM, Zabezhinskii MA, Piskunova TS, Popovich IG, Semenchenko AV. Metformin decelerates aging and development of mammary tumors in HER-2/neu transgenic mice. Bull Exp Biol Med. 2005;139(6):721–3. doi: 10.1007/s10517-005-0389-9. [DOI] [PubMed] [Google Scholar]
- 163.Henderson ST, Bonafe M, Johnson TE. daf-16 protects the nematode Caenorhabditis elegans during food deprivation. J Gerontol A Biol Sci Med Sci. 2006;61(5):444–60. doi: 10.1093/gerona/61.5.444. [DOI] [PubMed] [Google Scholar]
- 164.Hansen M, Taubert S, Crawford D, Libina N, Lee SJ, Kenyon C. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell. 2007;6(1):95–110. doi: 10.1111/j.1474-9726.2006.00267.x. [DOI] [PubMed] [Google Scholar]
- 165.Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14(10):885–90. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fay JR, Steele V, Crowell JA. Energy homeostasis and cancer prevention: the AMP-activated protein kinase. Cancer Prev Res (Phila) 2009;2(4):301–9. doi: 10.1158/1940-6207.CAPR-08-0166. [DOI] [PubMed] [Google Scholar]
- 167.Luo Z, Saha AK, Xiang X, Ruderman NB. AMPK, the metabolic syndrome and cancer. Trends Pharmacol Sci. 2005;26(2):69–76. doi: 10.1016/j.tips.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 168.Hardie DG. Role of AMP-activated protein kinase in the metabolic syndrome and in heart disease. FEBS Lett. 2008;582(1):81–9. doi: 10.1016/j.febslet.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 169.Sanchez-Cespedes M. A role for LKB1 gene in human cancer beyond the Peutz-Jeghers syndrome. Oncogene. 2007;26(57):7825–32. doi: 10.1038/sj.onc.1210594. [DOI] [PubMed] [Google Scholar]
- 170.Fenton H, Carlile B, Montgomery EA, Carraway H, Herman J, Sahin F, Su GH, Argani P. LKB1 protein expression in human breast cancer. Appl Immunohistochem Mol Morphol. 2006;14(2):146–53. doi: 10.1097/01.pai.0000176157.07908.20. [DOI] [PubMed] [Google Scholar]
- 171.Huang X, Wullschleger S, Shpiro N, McGuire VA, Sakamoto K, Woods YL, McBurnie W, Fleming S, Alessi DR. Important role of the LKB1-AMPK pathway in suppressing tumorigenesis in PTEN-deficient mice. Biochem J. 2008;412(2):211–21. doi: 10.1042/BJ20080557. [DOI] [PubMed] [Google Scholar]
- 172.Imamura K, Ogura T, Kishimoto A, Kaminishi M, Esumi H. Cell cycle regulation via p53 phosphorylation by a 5′-AMP activated protein kinase activator, 5-aminoimidazole- 4-carboxamide-1-beta-D-ribofuranoside, in a human hepatocellular carcinoma cell line. Biochem Biophys Res Commun. 2001;287(2):562–7. doi: 10.1006/bbrc.2001.5627. [DOI] [PubMed] [Google Scholar]
- 173.Xiang X, Saha AK, Wen R, Ruderman NB, Luo Z. AMP-activated protein kinase activators can inhibit the growth of prostate cancer cells by multiple mechanisms. Biochem Biophys Res Commun. 2004;321(1):161–7. doi: 10.1016/j.bbrc.2004.06.133. [DOI] [PubMed] [Google Scholar]
- 174.Swinnen JV, Beckers A, Brusselmans K, Organe S, Segers J, Timmermans L, Vanderhoydonc F, Deboel L, Derua R, Waelkens E, De Schrijver E, Van de Sande T, Noel A, Foufelle F, Verhoeven G. Mimicry of a cellular low energy status blocks tumor cell anabolism and suppresses the malignant phenotype. Cancer Res. 2005;65(6):2441–8. doi: 10.1158/0008-5472.CAN-04-3025. [DOI] [PubMed] [Google Scholar]
- 175.Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, Viollet B, Thompson CB. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67(14):6745–52. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- 176.Stapleton D, Mitchelhill KI, Gao G, Widmer J, Michell BJ, Teh T, House CM, Fernandez CS, Cox T, Witters LA, Kemp BE. Mammalian AMP-activated protein kinase subfamily. J Biol Chem. 1996;271(2):611–4. doi: 10.1074/jbc.271.2.611. [DOI] [PubMed] [Google Scholar]
- 177.Blazquez C, Geelen MJ, Velasco G, Guzman M. The AMP-activated protein kinase prevents ceramide synthesis de novo and apoptosis in astrocytes. FEBS Lett. 2001;489(2-3):149–53. doi: 10.1016/s0014-5793(01)02089-0. [DOI] [PubMed] [Google Scholar]
- 178.Lopez-Lopez C, Dietrich MO, Metzger F, Loetscher H, Torres-Aleman I. Disturbed cross talk between insulin-like growth factor I and AMP-activated protein kinase as a possible cause of vascular dysfunction in the amyloid precursor protein/presenilin 2 mouse model of Alzheimer’s disease. J Neurosci. 2007;27(4):824–31. doi: 10.1523/JNEUROSCI.4345-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Culmsee C, Monnig J, Kemp BE, Mattson MP. AMP-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. J Mol Neurosci. 2001;17(1):45–58. doi: 10.1385/JMN:17:1:45. [DOI] [PubMed] [Google Scholar]
- 180.Li J, Zeng Z, Viollet B, Ronnett GV, McCullough LD. Neuroprotective effects of adenosine monophosphate-activated protein kinase inhibition and gene deletion in stroke. Stroke. 2007;38(11):2992–9. doi: 10.1161/STROKEAHA.107.490904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Greco SJ, Sarkar S, Casadesus G, Zhu X, Smith MA, Ashford JW, Johnston JM, Tezapsidis N. Leptin inhibits glycogen synthase kinase-3beta to prevent tau phosphorylation in neuronal cells. Neurosci Lett. 2009;455(3):191–4. doi: 10.1016/j.neulet.2009.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ma TC, Buescher JL, Oatis B, Funk JA, Nash AJ, Carrier RL, Hoyt KR. Metformin therapy in a transgenic mouse model of Huntington’s disease. Neurosci Lett. 2007;411(2):98–103. doi: 10.1016/j.neulet.2006.10.039. [DOI] [PubMed] [Google Scholar]
- 183.Chen Y, Zhou K, Wang R, Liu Y, Kwak YD, Ma T, Thompson RC, Zhao Y, Smith L, Gasparini L, Luo Z, Xu H, Liao FF. Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer’s amyloid peptides via up-regulating BACE1 transcription. Proc Natl Acad Sci U S A. 2009;106(10):3907–12. doi: 10.1073/pnas.0807991106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Profenno LA, Porsteinsson AP, Faraone SV. Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biol Psychiatry. 67(6):505–12. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 185.Cheung WD, Hart GW. AMP-activated protein kinase and p38 MAPK activate O-GlcNAcylation of neuronal proteins during glucose deprivation. J Biol Chem. 2008;283(19):13009–20. doi: 10.1074/jbc.M801222200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Winder WW, Hardie DG. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol. 1999;277(1 Pt 1):E1–10. doi: 10.1152/ajpendo.1999.277.1.E1. [DOI] [PubMed] [Google Scholar]
- 187.Calvert JW, Gundewar S, Jha S, Greer JJ, Bestermann WH, Tian R, Lefer DJ. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes. 2008;57(3):696–705. doi: 10.2337/db07-1098. [DOI] [PubMed] [Google Scholar]
- 188.Bonnard C, Durand A, Vidal H, Rieusset J. Changes in adiponectin, its receptors and AMPK activity in tissues of diet-induced diabetic mice. Diabetes Metab. 2008;34(1):52–61. doi: 10.1016/j.diabet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 189.Wang S, Xu J, Song P, Viollet B, Zou MH. In vivo activation of AMP-activated protein kinase attenuates diabetes-enhanced degradation of GTP cyclohydrolase I. Diabetes. 2009;58(8):1893–901. doi: 10.2337/db09-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Xie Z, Zhang J, Wu J, Viollet B, Zou MH. Upregulation of mitochondrial uncoupling protein-2 by the AMP-activated protein kinase in endothelial cells attenuates oxidative stress in diabetes. Diabetes. 2008;57(12):3222–30. doi: 10.2337/db08-0610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 191.Li Q, Li J, Ren J. UCF-101 mitigates streptozotocin-induced cardiomyocyte dysfunction: role of AMPK. Am J Physiol Endocrinol Metab. 2009;297(4):E965–73. doi: 10.1152/ajpendo.00323.2009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 192.Bergeron R, Russell RR, 3rd, Young LH, Ren JM, Marcucci M, Lee A, Shulman GI. Effect of AMPK activation on muscle glucose metabolism in conscious rats. Am J Physiol. 1999;276(5 Pt 1):E938–44. doi: 10.1152/ajpendo.1999.276.5.E938. [DOI] [PubMed] [Google Scholar]
- 193.Mu J, Brozinick JT, Jr., Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell. 2001;7(5):1085–94. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- 194.Iglesias MA, Ye JM, Frangioudakis G, Saha AK, Tomas E, Ruderman NB, Cooney GJ, Kraegen EW. AICAR administration causes an apparent enhancement of muscle and liver insulin action in insulin-resistant high-fat-fed rats. Diabetes. 2002;51(10):2886–94. doi: 10.2337/diabetes.51.10.2886. [DOI] [PubMed] [Google Scholar]
- 195.Chan AY, Soltys CL, Young ME, Proud CG, Dyck JR. Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J Biol Chem. 2004;279(31):32771–9. doi: 10.1074/jbc.M403528200. [DOI] [PubMed] [Google Scholar]
- 196.Shibata R, Ouchi N, Kihara S, Sato K, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp-activated protein kinase signaling. J Biol Chem. 2004;279(27):28670–4. doi: 10.1074/jbc.M402558200. [DOI] [PubMed] [Google Scholar]
- 197.Sasaki I, Tomizawa M, Kudo B, Ogasawara M. [Study on the typing and epidemiology of a mass occurrence of Shigella sonnei] Nihon Densenbyo Gakkai Zasshi. 1967;41(3):115–21. [PubMed] [Google Scholar]
- 198.Russell RR, 3rd, Bergeron R, Shulman GI, Young LH. Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am J Physiol. 1999;277(2 Pt 2):H643–9. doi: 10.1152/ajpheart.1999.277.2.H643. [DOI] [PubMed] [Google Scholar]
- 199.Xing Y, Musi N, Fujii N, Zou L, Luptak I, Hirshman MF, Goodyear LJ, Tian R. Glucose metabolism and energy homeostasis in mouse hearts overexpressing dominant negative alpha2 subunit of AMP-activated protein kinase. J Biol Chem. 2003;278(31):28372–7. doi: 10.1074/jbc.M303521200. [DOI] [PubMed] [Google Scholar]
- 200.Russell RR, 3rd, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, Giordano FJ, Mu J, Birnbaum MJ, Young LH. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004;114(4):495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cacicedo JM, Yagihashi N, Keaney JF, Jr., Ruderman NB, Ido Y. AMPK inhibits fatty acid-induced increases in NF-kappaB transactivation in cultured human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2004;324(4):1204–9. doi: 10.1016/j.bbrc.2004.09.177. [DOI] [PubMed] [Google Scholar]
- 202.Dixit M, Bess E, Fisslthaler B, Hartel FV, Noll T, Busse R, Fleming I. Shear stress-induced activation of the AMP-activated protein kinase regulates FoxO1a and angiopoietin-2 in endothelial cells. Cardiovasc Res. 2008;77(1):160–8. doi: 10.1093/cvr/cvm017. [DOI] [PubMed] [Google Scholar]
- 203.Hattori Y, Nakano Y, Hattori S, Tomizawa A, Inukai K, Kasai K. High molecular weight adiponectin activates AMPK and suppresses cytokine-induced NF-kappaB activation in vascular endothelial cells. FEBS Lett. 2008;582(12):1719–24. doi: 10.1016/j.febslet.2008.04.037. [DOI] [PubMed] [Google Scholar]
- 204.Schulz E, Dopheide J, Schuhmacher S, Thomas SR, Chen K, Daiber A, Wenzel P, Munzel T, Keaney JF., Jr. Suppression of the JNK pathway by induction of a metabolic stress response prevents vascular injury and dysfunction. Circulation. 2008;118(13):1347–57. doi: 10.1161/CIRCULATIONAHA.108.784298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Colombo SL, Moncada S. AMPKalpha1 regulates the antioxidant status of vascular endothelial cells. Biochem J. 2009;421(2):163–9. doi: 10.1042/BJ20090613. [DOI] [PubMed] [Google Scholar]
- 206.Alba G, El Bekay R, Alvarez-Maqueda M, Chacon P, Vega A, Monteseirin J, Santa Maria C, Pintado E, Bedoya FJ, Bartrons R, Sobrino F. Stimulators of AMP-activated protein kinase inhibit the respiratory burst in human neutrophils. FEBS Lett. 2004;573(1-3):219–25. doi: 10.1016/j.febslet.2004.07.077. [DOI] [PubMed] [Google Scholar]
- 207.Ceolotto G, Gallo A, Papparella I, Franco L, Murphy E, Iori E, Pagnin E, Fadini GP, Albiero M, Semplicini A, Avogaro A. Rosiglitazone reduces glucose-induced oxidative stress mediated by NAD(P)H oxidase via AMPK-dependent mechanism. Arterioscler Thromb Vasc Biol. 2007;27(12):2627–33. doi: 10.1161/ATVBAHA.107.155762. [DOI] [PubMed] [Google Scholar]
- 208.Nagata D, Takeda R, Sata M, Satonaka H, Suzuki E, Nagano T, Hirata Y. AMP-activated protein kinase inhibits angiotensin II-stimulated vascular smooth muscle cell proliferation. Circulation. 2004;110(4):444–51. doi: 10.1161/01.CIR.0000136025.96811.76. [DOI] [PubMed] [Google Scholar]
- 209.Stuck BJ, Lenski M, Bohm M, Laufs U. Metabolic switch and hypertrophy of cardiomyocytes following treatment with angiotensin II are prevented by AMP-activated protein kinase. J Biol Chem. 2008;283(47):32562–9. doi: 10.1074/jbc.M801904200. [DOI] [PubMed] [Google Scholar]
- 210.Chen Z, Peng IC, Sun W, Su MI, Hsu PH, Fu Y, Zhu Y, DeFea K, Pan S, Tsai MD, Shyy JY. AMP-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase Ser633. Circ Res. 2009;104(4):496–505. doi: 10.1161/CIRCRESAHA.108.187567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Michell BJ, Chen Z, Tiganis T, Stapleton D, Katsis F, Power DA, Sim AT, Kemp BE. Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase C and the cAMP-dependent protein kinase. J Biol Chem. 2001;276(21):17625–8. doi: 10.1074/jbc.C100122200. [DOI] [PubMed] [Google Scholar]
- 212.Nagata D, Mogi M, Walsh K. AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J Biol Chem. 2003;278(33):31000–6. doi: 10.1074/jbc.M300643200. [DOI] [PubMed] [Google Scholar]
- 213.Thors B, Halldorsson H, Thorgeirsson G. Thrombin and histamine stimulate endothelial nitric-oxide synthase phosphorylation at Ser1177 via an AMPK mediated pathway independent of PI3K-Akt. FEBS Lett. 2004;573(1-3):175–80. doi: 10.1016/j.febslet.2004.07.078. [DOI] [PubMed] [Google Scholar]
- 214.Fleming I, Fisslthaler B, Dixit M, Busse R. Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. J Cell Sci. 2005;118(Pt 18):4103–11. doi: 10.1242/jcs.02541. [DOI] [PubMed] [Google Scholar]
- 215.Fisslthaler B, Fleming I, Keseru B, Walsh K, Busse R. Fluid shear stress and NO decrease the activity of the hydroxy-methylglutaryl coenzyme A reductase in endothelial cells via the AMP-activated protein kinase and FoxO1. Circ Res. 2007;100(2):e12–21. doi: 10.1161/01.RES.0000257747.74358.1c. [DOI] [PubMed] [Google Scholar]
- 216.Morrow VA, Foufelle F, Connell JM, Petrie JR, Gould GW, Salt IP. Direct activation of AMP-activated protein kinase stimulates nitric-oxide synthesis in human aortic endothelial cells. J Biol Chem. 2003;278(34):31629–39. doi: 10.1074/jbc.M212831200. [DOI] [PubMed] [Google Scholar]
- 217.Cheng KK, Lam KS, Wang Y, Huang Y, Carling D, Wu D, Wong C, Xu A. Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes. 2007;56(5):1387–94. doi: 10.2337/db06-1580. [DOI] [PubMed] [Google Scholar]
- 218.Reihill JA, Ewart MA, Hardie DG, Salt IP. AMP-activated protein kinase mediates VEGF-stimulated endothelial NO production. Biochem Biophys Res Commun. 2007;354(4):1084–8. doi: 10.1016/j.bbrc.2007.01.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Boyle JG, Logan PJ, Ewart MA, Reihill JA, Ritchie SA, Connell JM, Cleland SJ, Salt IP. Rosiglitazone stimulates nitric oxide synthesis in human aortic endothelial cells via AMP-activated protein kinase. J Biol Chem. 2008;283(17):11210–7. doi: 10.1074/jbc.M710048200. [DOI] [PubMed] [Google Scholar]
- 220.Zou MH, Kirkpatrick SS, Davis BJ, Nelson JS, Wiles W. G. t., Schlattner U, Neumann D, Brownlee M, Freeman MB, Goldman MH. Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo. Role of mitochondrial reactive nitrogen species. J Biol Chem. 2004;279(42):43940–51. doi: 10.1074/jbc.M404421200. [DOI] [PubMed] [Google Scholar]
- 221.Davis BJ, Xie Z, Viollet B, Zou MH. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes. 2006;55(2):496–505. doi: 10.2337/diabetes.55.02.06.db05-1064. [DOI] [PubMed] [Google Scholar]
- 222.Zhang J, Xie Z, Dong Y, Wang S, Liu C, Zou MH. Identification of nitric oxide as an endogenous activator of the AMP-activated protein kinase in vascular endothelial cells. J Biol Chem. 2008;283(41):27452–61. doi: 10.1074/jbc.M802578200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 223.Webler AC, Michaelis UR, Popp R, Barbosa-Sicard E, Murugan A, Falck JR, Fisslthaler B, Fleming I. Epoxyeicosatrienoic acids are part of the VEGF-activated signaling cascade leading to angiogenesis. Am J Physiol Cell Physiol. 2008;295(5):C1292–301. doi: 10.1152/ajpcell.00230.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ouchi N, Shibata R, Walsh K. AMP-activated protein kinase signaling stimulates VEGF expression and angiogenesis in skeletal muscle. Circ Res. 2005;96(8):838–46. doi: 10.1161/01.RES.0000163633.10240.3b. [DOI] [PubMed] [Google Scholar]
- 225.Leick L, Hellsten Y, Fentz J, Lyngby SS, Wojtaszewski JF, Hidalgo J, Pilegaard H. PGC-1alpha mediates exercise-induced skeletal muscle VEGF expression in mice. Am J Physiol Endocrinol Metab. 2009;297(1):E92–103. doi: 10.1152/ajpendo.00076.2009. [DOI] [PubMed] [Google Scholar]
- 226.Katakam PV, Ujhelyi MR, Hoenig M, Miller AW. Metformin improves vascular function in insulin-resistant rats. Hypertension. 2000;35(1 Pt 1):108–12. doi: 10.1161/01.hyp.35.1.108. [DOI] [PubMed] [Google Scholar]
- 227.Sartoretto JL, Melo GA, Carvalho MH, Nigro D, Passaglia RT, Scavone C, Cuman RK, Fortes ZB. Metformin treatment restores the altered microvascular reactivity in neonatal streptozotocin-induced diabetic rats increasing NOS activity, but not NOS expression. Life Sci. 2005;77(21):2676–89. doi: 10.1016/j.lfs.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 228.Blattler SM, Rencurel F, Kaufmann MR, Meyer UA. In the regulation of cytochrome P450 genes, phenobarbital targets LKB1 for necessary activation of AMP-activated protein kinase. Proc Natl Acad Sci U S A. 2007;104(3):1045–50. doi: 10.1073/pnas.0610216104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Rencurel F, Foretz M, Kaufmann MR, Stroka D, Looser R, Leclerc I, da Silva Xavier G, Rutter GA, Viollet B, Meyer UA. Stimulation of AMP-activated protein kinase is essential for the induction of drug metabolizing enzymes by phenobarbital in human and mouse liver. Mol Pharmacol. 2006;70(6):1925–34. doi: 10.1124/mol.106.029421. [DOI] [PubMed] [Google Scholar]
- 230.Goirand F, Solar M, Athea Y, Viollet B, Mateo P, Fortin D, Leclerc J, Hoerter J, Ventura-Clapier R, Garnier A. Activation of AMP kinase alpha1 subunit induces aortic vasorelaxation in mice. J Physiol. 2007;581(Pt 3):1163–71. doi: 10.1113/jphysiol.2007.132589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Rubin LJ, Magliola L, Feng X, Jones AW, Hale CC. Metabolic activation of AMP kinase in vascular smooth muscle. J Appl Physiol. 2005;98(1):296–306. doi: 10.1152/japplphysiol.00075.2004. [DOI] [PubMed] [Google Scholar]
- 232.McCarty MF. AMPK activation as a strategy for reversing the endothelial lipotoxicity underlying the increased vascular risk associated with insulin resistance syndrome. Med Hypotheses. 2005;64(6):1211–5. doi: 10.1016/j.mehy.2004.01.042. [DOI] [PubMed] [Google Scholar]
- 233.Brito PM, Devillard R, Negre-Salvayre A, Almeida LM, Dinis TC, Salvayre R, Auge N. Resveratrol inhibits the mTOR mitogenic signaling evoked by oxidized LDL in smooth muscle cells. Atherosclerosis. 2009;205(1):126–34. doi: 10.1016/j.atherosclerosis.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 234.Zou MH, Hou XY, Shi CM, Kirkpatick S, Liu F, Goldman MH, Cohen RA. Activation of 5′-AMP-activated kinase is mediated through c-Src and phosphoinositide 3-kinase activity during hypoxia-reoxygenation of bovine aortic endothelial cells. Role of peroxynitrite. J Biol Chem. 2003;278(36):34003–10. doi: 10.1074/jbc.M300215200. [DOI] [PubMed] [Google Scholar]
- 235.Zhang M, Dong Y, Xu J, Xie Z, Wu Y, Song P, Guzman M, Wu J, Zou MH. Thromboxane receptor activates the AMP-activated protein kinase in vascular smooth muscle cells via hydrogen peroxide. Circ Res. 2008;102(3):328–37. doi: 10.1161/CIRCRESAHA.107.163253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Xie Z, Dong Y, Scholz R, Neumann D, Zou MH. Phosphorylation of LKB1 at serine 428 by protein kinase C-zeta is required for metformin-enhanced activation of the AMP-activated protein kinase in endothelial cells. Circulation. 2008;117(7):952–62. doi: 10.1161/CIRCULATIONAHA.107.744490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Choi HC, Song P, Xie Z, Wu Y, Xu J, Zhang M, Dong Y, Wang S, Lau K, Zou MH. Reactive nitrogen species is required for the activation of the AMP-activated protein kinase by statin in vivo. J Biol Chem. 2008;283(29):20186–97. doi: 10.1074/jbc.M803020200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 238.Viollet B, Mounier R, Leclerc J, Yazigi A, Foretz M, Andreelli F. Targeting AMP-activated protein kinase as a novel therapeutic approach for the treatment of metabolic disorders. Diabetes Metab. 2007;33(6):395–402. doi: 10.1016/j.diabet.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 239.Zang M, Zuccollo A, Hou X, Nagata D, Walsh K, Herscovitz H, Brecher P, Ruderman NB, Cohen RA. AMP-activated protein kinase is required for the lipid-lowering effect of metformin in insulin-resistant human HepG2 cells. J Biol Chem. 2004;279(46):47898–905. doi: 10.1074/jbc.M408149200. [DOI] [PubMed] [Google Scholar]
- 240.Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, Cohen RA. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006;55(8):2180–91. doi: 10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]
- 241.Bailey CJ. Metformin: a multitasking medication. Diab Vasc Dis Res. 2008;5(3):156. doi: 10.3132/dvdr.2008.026. [DOI] [PubMed] [Google Scholar]
- 242.Matsumoto T, Noguchi E, Ishida K, Kobayashi T, Yamada N, Kamata K. Metformin normalizes endothelial function by suppressing vasoconstrictor prostanoids in mesenteric arteries from OLETF rats, a model of type 2 diabetes. Am J Physiol Heart Circ Physiol. 2008;295(3):H1165–H1176. doi: 10.1152/ajpheart.00486.2008. [DOI] [PubMed] [Google Scholar]