Abstract
Background
New drugs are routinely screened for acute IKr blocking properties thought to predict QT prolonging and arrhythmogenic liability. However, recent data suggest that chronic (hours) drug exposure to PI3 kinase (PI3K) inhibitors used in cancer can prolong QT by inhibiting potassium currents and increasing late sodium current (INa-L) in cardiomyocytes. We tested the extent to which IKr blockers with known QT liability generate arrhythmias through this pathway.
Methods and Results
Acute exposure to dofetilide, an IKr blocker without other recognized electropharmacologic actions, produced no change in ion currents or action potentials in adult mouse cardiomyocytes, which lack IKr. By contrast, 2–48 hours’ exposure to the drug generated arrhythmogenic afterdepolarizations and up to 15-fold increases in INa-L. Including PIP3, a downstream effector for the PI3K pathway, in the pipette inhibited these effects. INa-L was also increased, and inhibitable by PIP3, with hours of dofetilide exposure in human iPSC-derived cardiomyocytes and in CHO cells transfected with SCN5A, encoding INa. Cardiomyocytes from dofetilide-treated mice similarly demonstrated increased INa-L and afterdepolarizations. Other agents with variable IKr blocking potencies and arrhythmia liability produced a range of effects on INa-L, from marked increases (E-4031, d-sotalol, thioridazine, erythromycin) to little or no effect (haloperidol, moxifloxacin, verapamil).
Conclusions
Some but not all drugs designated as arrhythmogenic IKr blockers can generate arrhythmias by augmenting INa-L through the PI3K pathway. These data identify a potential mechanism for individual susceptibility to proarrhythmia and highlight the need for a new paradigm to screen drugs for QT prolonging and arrhythmogenic liability.
Keywords: Late sodium current, IKr block, arrhythmogenic, phosphoinositide 3-kinase (PI3K) inhibition
Introduction
Exaggerated QT prolongation and increased risk for the polymorphic ventricular tachycardia Torsades de Pointes (TdP), the drug-induced long QT syndrome (diLQTS), has been a major cause for drug relabeling and drug withdrawals.1,2 The adverse effect occurs in 1–3% of patients exposed to QT prolonging antiarrhythmics such as sotalol or dofetilide, and less commonly during treatment with multiple “non-cardiovascular” drugs, including certain antibiotics, antipsychotics, and methadone. Multiple lines of evidence support the view that block of the rapid component of the cardiac delayed rectifier potassium current (IKr) is the common mechanism: these include the findings that culprit drugs inhibit IKr and parallels between genetic loss of IKr function in type 2 congenital long QT syndrome due to mutations in KCNH2 (that reduce IKr) and the clinical features of diLQTS, such as increased risk in women or with hypokalemia. Indeed, the links between IKr block and diLQTS risk have become ingrained in drug development, since candidate molecules exhibiting potent IKr block in vitro are rarely developed and virtually all new drugs undergo a clinical “thorough QT study” comparing the extent of QT prolongation with that observed with a single dose of the antibiotic moxifloxacin, which blocks IKr, produces consistent but very modest QT prolongation, and has been associated with diLQTS only very rarely.3,4
The label for the tyrosine kinase inhibitor nilotinib carries a warning because of a potential risk for diLQTS.5 The data supporting this warning include small increases in QTc during therapy,6 and cases of TdP reported to the FDA.5 Recognizing that tyrosine kinase activates downstream signaling via phosphoinositide 3-kinase (PI3K), Lu et al7 tested the hypothesis that nilotinib and related anticancer drugs prolong action potentials (APs) and are arrhythmogenic by inhibiting PI3K. They found that chronic (hours) but not acute drug exposure prolonged canine cardiomyocyte APs, and that this effect was reversed by intracellular dialysis with phosphatidylinositol 3,4,5-trisphosphate (PIP3) a downstream effector of PI3K. They also observed that AP prolongation was mediated by both decreases in IKr and a second delayed rectifier (IKs), as well as increases in sodium current recorded several hundred milliseconds after a step depolarization and thus termed “late” sodium current, INa-L. They reported similar findings with the antihistamine terfenadine, a potent IKr blocker (with known effects on calcium and sodium channels) withdrawn from the US market because of diLQTS risk.
These provocative findings raise the question of whether this mechanism applies to other drugs with known diLQTS liability and specifically to drugs whose sole recognized electrophysiologic action is IKr block. Accordingly, the approach we adopted here was to determine the effects of dofetilide, an IKr blocker thought to be devoid of other significant electropharmacologic effects,8 as well as those of other IKr blockers with a spectrum of TdP risk, including the clinical reference agent moxifloxacin. We studied both acute and chronic drug exposure in adult mouse cardiomyocytes, which unlike canine cardiomyocytes lack IKr,9 as well as in CHO cells transfected with SCN5A that underlies the human cardiac sodium current (INa). Our findings in these cells and in human induced pluripotent stem cell derived cardiomyocytes (hiPSC-CMs) lend strong support to the concept that chronic dofetilide exposure strikingly increases INa-L and can thereby cause arrhythmias. Further, the effect was observed with some but not all other drugs tested, and not with moxifloxacin, a finding supporting the idea that this newly-described arrhythmogenic action contributes to the variability with which IKr blockers cause diLQTS.
Materials and Methods
Methods for FuGENE6-mediated SCN5A channel expression and cell transfection, isolation of mouse ventricular cardiomyocytes, sodium current and action potential recordings, and Western blotting are described in the on-line supplement and are similar to those reported previously.10–13
Reprogramming and generating human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs)
hiPSC-CM lines were developed from subjects without manifest cardiac phenotypes using the episomal vector method.14 Briefly, episomal vectors were transfected into fibroblasts via nucleofection. Cells were then plated onto Matrigel coated plates. Induced pluripotent stem cell (iPSC)-like colonies were picked up at ~Day 20 post-transfection. The matrix sandwich method was used to generate human cardiomyocytes (iPSC-CM) from normal human iPSCs.15 Single iPSCs were plated onto Matrigel coated 6-well plates, and growth factors (Activin A, BMP4 and bFGF) and Matrigel were added sequentially to differentiate the iPSCs into cardiomyocytes. hiPSC-CMs were then re-plated onto fibronectin coated plates and incubated at 37°C for 30–35 days post-induction. Spontaneously-beating clusters of hiPSC-CMs were used for action potential recordings in current-clamp mode. Single cardiomyocytes were used for the ion current recordings in voltage-clamp mode after brief trypsinization.
Chemicals
Tetrodotoxin (TTX), ranolazine, dofetilide, haloperidol, moxifloxacin, 4-aminopyridine (4-AP), erythromycin, thioridazine, verapamil, phosphatidylinositol 3,4,5-trisphosphate (PIP3), ATX-II, and LY294002 were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). E-4031 and d-sotalol were obtained from Tocris Bioscience (Minneapolis, MN, USA) and Bristol-Myers Squibb Co., respectively. Stock solutions for the tested drugs were prepared according to the vendors’ instructions and then diluted for studies as needed.
Statistical analysis
Results are presented as mean±SEM or median and IQR (inter-quartile range at the 25th and 75th percentiles). For the data showing a normal distribution, t-tests or analysis of variance (ANOVA) were used. A t-test assuming unequal variance was used for comparisons of two groups with unequal variances. For experiments with outlying data points or deviations from normality, the nonparametric Wilcoxon Signed Rank Sum (for paired data) and Mann-Whitney U test (for unpaired data) tests were used for comparisons. To account for multiple comparisons, a Bonferroni correction was applied, as noted in the respective figure legends. A Bonferroni corrected P<0.05 was considered statistically significant. Statistical analyses were carried out with SPSS software version 22 (IBM, NY, USA), SAS software version 6.1.7601 (SAS Institute, Cary, NC, USA) and OriginPro 8.5.1 (OriginLab, Northampton, MA, USA).
Results
Comparison of the effects of acute dofetilide and 4-aminopyridine on action potentials and potassium current in adult mouse ventricular myocytes (Figure 1)
Figure 1.
Acute and chronic effects of dofetilide and 4-aminopyridine (4-AP) on action potentials and potassium current in mouse. A. After an acute (15 minute) exposure to dofetilide (dof), mouse cardiomyocyte action potentials were superimposable on those recorded in control. B. Acute exposure to 4-AP prolonged action potential duration. C. Effect of dofetilide and 4-AP on potassium currents. There was no change with dofetilide but a pronounced suppression with 4-AP, as expected from previous reports. Note that the signature of IKr, a deactivating tail current, is absent in these cells and that the major current is the transient outward potassium current (ITO). D. Summary of the effects of dofetilide and 4-AP on ITO (n=6–7). P-values are from t-tests assuming unequal variance, and are corrected for 3 statistical tests. These experiments were conducted in ventricular cardiomyocytes isolated from female C57 mice.
We first confirmed that the dofetilide target IKr does not play a significant role in repolarization of adult mouse cardiomyocytes. Figure 1A shows that acute exposure to 1 µM dofetilide had no effect on action potential durations in adult mouse cardiomyocytes. By contrast, the prototypical transient outward current (ITO) blocker 4-aminopyridine (4-AP, 500 µM) produced the expected AP prolongation (Figure 1B). In voltage clamp, dofetilide produced no effect on outward potassium current in these cells (Figure 1C), whereas 4-AP suppressed ITO as expected (Figures 1C and 1D).
Chronic exposure to dofetilide prolongs action potentials (APs) and generates abnormal automaticity in mouse cardiomyocytes (Figure 2)
Figure 2.
Effect of exposure to dofetilide for 5 hours in mouse cardiomyocytes. A. Chronic dofetilide prolonged action potentials at a stimulation rate of 1 Hz. An example is shown in the top panel, and summary data on APD at 50% and 90% repolarization (APD50 and APD90) for acute exposure to dofetilide and 4-AP (from Figure 1) and 5-hour exposure to dofetilide are shown below (n=6–8 each). P-values are from t-tests assuming unequal variance, and are corrected for 4 statistical tests. B. Example of action potentials recorded at a slow stimulation rate (0.1 Hz) 5 hours after isolation with no drug exposure. No abnormalities are seen. Traces recorded immediately after break-in (labeled 1st min) and 5 minutes later (5th min) are shown in this panel and in panels C and D. C. Example of action potentials recorded 5 hours after isolation with exposure to dofetilide. The insets show action potential prolongation and afterdepolarizations (small arrows). D. Example of action potentials recorded 5 hours after isolation with exposure to dofetilide, and with phosphatidylinositol 3,4,5-trisphosphate (PIP3) in the pipette. The insets show action potential prolongation at minute 1, and then action potential shortening by minute 5. There are no afterdepolarizations. E. Summary data showing frequency of afterdepolarizations in individual experiments. A t-test assuming unequal variance, and adjusted for 3 tests, was used for comparisons between groups.
In contrast to the lack of acute effects of dofetilide, we observed striking AP prolongation (Figure 2A) when adult mouse cardiomyocytes were exposed to the drug for 5 hours. Figure 2A (bottom) summarizes these results and shows that the effects of chronic dofetilide exposure on APD50 and APD90 were similar to those of acute 4-AP.
Figure 2B shows a train of action potentials recorded 5 hours after cell isolation and driven at 0.1 Hz in the absence of drug exposure. With exposure to dofetilide for 5 hours, triggered beats arising from early and delayed afterdepolarizations (EADs and DADs) were regularly observed; an example is shown in Figure 2C. Including PIP3 in the pipette solution in cells exposed to dofetilide for 5 hours reversed AP prolongation (Figure 2D) and inhibited afterdepolarizations; as reported by others, this effect was very rapid.7,16 Action potentials recorded from control cells with PIP3 in the pipette were no different from action potentials recorded without PIP3 in the pipette. Figure 2E summarizes the frequency of afterdepolarizations in cells studied ≥5 hr after isolation (controls, 0/15), cells exposed to dofetilide for 5 hr after isolation (15/19), and cells exposed to dofetilide for 5 hr after isolation and studied with PIP3 in the pipette (2/15). No afterdepolarizations were observed in cells (n=3) exposed to 100 µM of the IKr blocking antibiotic moxifloxacin for 5 hours prior to study.
Chronic exposure to dofetilide and some, but not all, other IKr blockers enhances late sodium current (INa-L) in adult mouse ventricular myocytes and CHO cells (Figures 3 and 4)
Figure 3.
Chronic dofetilide exposure increases late sodium current in mouse ventricular myocytes. A. Family of potassium currents in mouse ventricular myocytes 5 hours after isolation in the absence of drug (control, left) or the presence of dofetilide (right). The summary current-voltage relations (n=6 each) are shown below. There was no effect of dofetilide on potassium current in these cells. B. Examples of late sodium current recorded 5 hours after isolation in the absence of drug (control), in the presence of dofetilide, or in the presence of dofetilide with PIP3 in the pipette. C. Individual and summary data. PIP3 almost completely inhibited the augmentation produced by dofetilide (data after 200 msec at −30 mV; n=6–10 each). P-values were computed using a t-test assuming unequal variances, and are adjusted for 3 statistical tests.
Figure 4.
Augmented dofetilide-induced late sodium current (INa-L) in CHO cells. A. Time-dependence of dofetilide effect (n=6–8 each). Median and inter-quartile ranges are shown in the graph. Exposure to drug caused a time-dependent increase in INa-L that was statistically significant as early as 2 hours. The figure also shows that there was no effect on INa-L of including PIP3 in the pipette under control conditions, but that at 5 and 48 hours, significant inhibition of INa-L was seen. P-values were computed using a Mann-Whitney U test, and are adjusted for 10 statistical tests. B. Concentration dependence of the dofetilide effect, yielding an IC50 of 103.4±5.4 nM (n=6–8 per data point). These data were generated with 48 hour drug exposures. There was a statistically significant increase in INa-L with even 10 nM (p<0.05). C. E-4031 and d-sotalol, previously considered to be specific IKr blockers, also increased INa-L as did erythromycin and thioridazine. By contrast, there was only a minor effect of haloperidol and there was no effect of either moxifloxacin or verapamil. Median and inter-quartile ranges are shown in the graph (Mann-Whitney U test, adjusted for 17 tests). D. Increased INa-L and peak INa induced by 48 hour exposure to dofetilide were both blocked by the selective sodium channel toxin blocker tetrodotoxin (TTX), and the effect was greater on INa-L (bottom right) than on peak current (bottom left). A paired t-test assuming unequal variance was used for data comparisons.
While 5 hours of exposure to dofetilide increased AP duration and generated abnormal automaticity, outward potassium currents were unchanged and remained dofetilide-insensitive (Figure 3A), indicating that the dofetilide effect on action potentials shown in Figure 2 could not be attributed to a change in potassium current.
Figures 3B and 3C show that INa-L in mouse ventricular myocytes was strikingly increased by 5 hours of exposure to dofetilide and that the effect was reversible by including PIP3 in the pipette. Basal INa-L at −30 mV in untreated control myocytes was very small, 0.17±0.01 pA/pF (n=10). However, in cells exposed to 1 µM dofetilide for 5 hours, there was a ~7-fold enhancement of INa-L to 1.2±0.1 pA/pF (n=8, P<0.01 vs other groups). When PIP3 was included in the pipette solution, the amplitude of INa-L in dofetilide-treated cells was identical to that in untreated cells. Importantly, we observed no effect of pipette PIP3 on INa-L stimulated by a low concentration (3 nM) of the sea anemone toxin ATX-II that destabilizes fast inactivation and increases INa-L:17,18 at −30 mV, INa-L was 713±136 pA (without PIP3, n=4) vs 688±216 pA (with PIP3, p>0.05, n=4). These data indicate that PIP3 is not a general inhibitor of augmented INa-L.
The effect of chronic dofetilide on INa-L was also seen in SCN5A-transfected CHO cells, and was concentration- and time-dependent (Figure 4). Time-dependence is shown in Figure 4A: there was no increase in late current within 30 minutes after exposure to 1 µM dofetilide, as compared to controls, in SCN5A-transfected CHO cells. However, at all times points starting at 2 hours of exposure, there was a significant increase in late current (median peak current of 0.30% versus 0.55% at 2 hours for controls versus treated, respectively) that persisted through all subsequent time points (median peak current of 4.0% at 48 hours exposure). Figure 4A also shows that including PIP3 in the pipette solution nearly completely attenuated the dofetilide-enhanced late current in these cells. The effect on INa-L of 48 hour exposure to varying dofetilide concentrations is shown in Figure 4B, yielding a 50% inhibitory concentration (IC50) of 103.4±5.4 nM: an increase was evident with even low concentrations (10 nM) of the drug.
We also examined the effects on INa-L of multiple IKr blockers that have been associated with a spectrum of diLQTS risk under the same conditions (48 hr exposure in SCN5A-transfected CHO cells; Figure 4C and Supplemental Figure 1). Like dofetilide, the potent and specific IKr blockers E-4031 and d-sotalol (methanesulfonanilides like dofetilide) also increased INa-L. By contrast, the clinical reference agent moxifloxacin (100 µM) had no effect on INa-L under these conditions, nor did the calcium channel blocker verapamil (1 µM), which is also known to block IKr but is not associated with TdP risk.19,20 However, other drugs associated with TdP risk and with variable IKr potencies (and structurally unrelated to dofetilide) did increase INa-L. These included the antibiotic erythromycin (at 100 µM), which blocks IKr at high concentrations (IC50 values of 50–1400 µM)21,22 in vitro but can cause occasionally TdP in humans; thioridazine (1 µM), a potent IKr blocker with IC50 values of 0.2–1.25 µM23,24 and among the most common antipsychotics causing TdP;25 and haloperidol (1 µM), another antipsychotic associated with TdP.
Figure 4D shows that the increased late current is blocked by the sodium channel specific inhibitor TTX (5 µM), confirming that this is a sodium current; 30 µM TTX completely suppressed both peak and late INa. Similarly, the INa-L selective blocker ranolazine (10 µM) completely inhibited INa-L in cells chronically exposed to dofetilide, E-4031 or d-sotalol, while peak INa was less affected (Supplemental Figure 2).
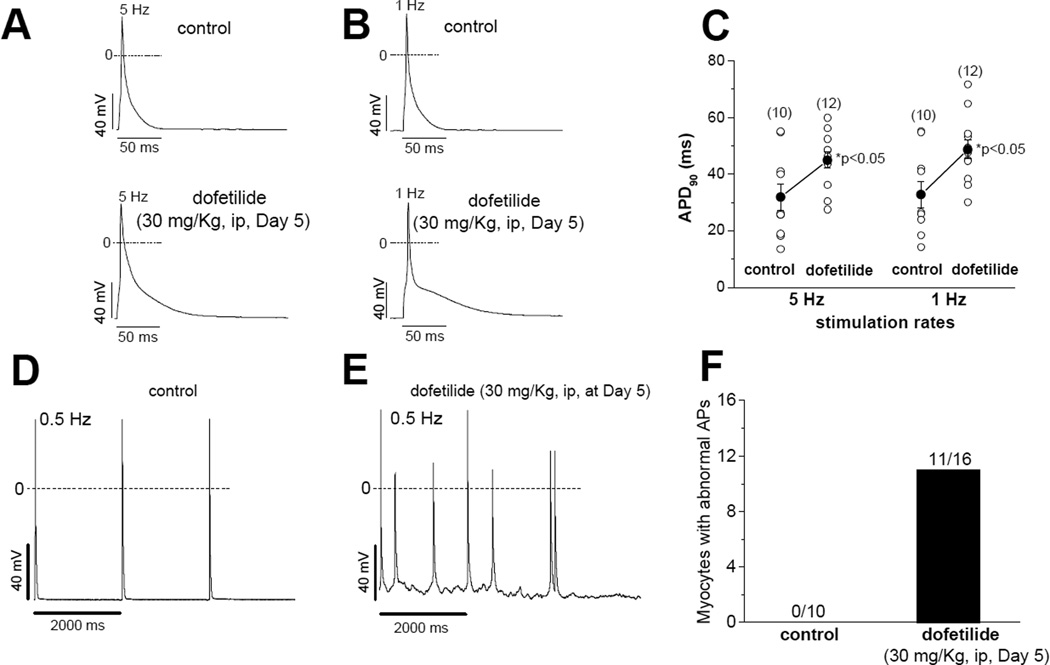
Action potential duration and late current are increased after dofetilide administration in vivo (Figure 5)
Figure 5.
Action potential duration and late current are increased after dofetilide administration in vivo. A–C. Representative action potentials (A and B) and summary data (C) at 5 and 1 Hz in mouse ventricular myocytes recorded at day 5 after dofetilide (30 mg/Kg, intraperitoneally). T-tests (equal variance) were used for data comparisons. D–F. Representative action potentials (D and E) and summary data (F) at a slower stimulation rate (0.5 Hz). The data were collected from six female mice (3 for control and 3 for dofetilide). The numbers of cells in each data group are shown in panels C and F.
In this set of experiments, three female mice were injected with a single large dose of dofetilide (30 mg/Kg intraperitoneally) and ventricular myocytes were isolated and studied 5 days later. These cells displayed striking action potential prolongation and afterdepolarizations at slow stimulation rates (Figure 5), effects not seen in three uninjected mice. Supplemental Figure 3 shows that these myocytes displayed increases in INa-L that were similar to those observed in the 5 hour in vitro exposure described above.
Chronic dofetilide enhanced INa-L in hiPSC-CMs (Figure 6)
Figure 6.
Five hour drug exposures to cardiomyocytes derived from human induced pluripotent stem cells (hiPSC-CMs). A. An example of a small INa-L in these cells. B. An example of current recorded after exposure to dofetilide. C. Effect of including PIP3 in the pipette solution in a dofetilide-exposed cell. The first and 20th traces during stimuli at 0.2 Hz are shown. D. An example of a current recorded after exposure to moxifloxacin. E. Summary data. Median and inter-quartile ranges are shown in the graph. The Mann-Whitney U test, adjusted for 4 statistical tests, was used for data comparisons. INa-L was recorded after 200 msec at −30 mV (n=6–8 each). F. Examples of the effects of acute and chronic (5 hours) dofetilide on spontaneous ventricular-like action potentials in hiPSC-CMs.
The development of methods to generate cardiomyocytes from hiPSCs allowed us to determine whether the results reported here in CHO cells and in mouse cardiomyocytes extend to human cells. First, we determined whether INa-L can be recorded in hiPSC-CMs. Mean cell size at the time of study was 46.5±3.4 pF (n=10). Using the solutions described in the Methods section (extracellular sodium concentration of 135 mmol/L), we detected a small INa-L (Figure 6A), with a median amplitude of 0.26 (IQR: 0.20 – 0.30 pA/pF; n=8). Adding dofetilide (1 µM) to the medium for 5 hours resulted in a 4-fold increase in amplitude (Figure 6B), to 1.65 (IQR: 1.35 – 2.40 pA/pF; n=8, P<0.001, and when PIP3 was included in the pipette solution, the current (0.29; IQR: 0.22 – 0.47 pA/pF; Figure 6C, n=6) was similar to that seen in untreated cells, as in the mouse cardiomyocytes and in CHO cells. By contrast, and also consistent with the mouse data, moxifloxacin (100 µM) did not increase INa-L (Figure 6D, 0.24, IQR: 0.19 – 0.37 pA/pF; n=6; p>0.05). The effects of drug exposure on INa-L in hiPSC-CMs are summarized in Figure 6E. As with other IKr blockers studied in iPSC-CMs,26,27 acute dofetilide markedly prolonged AP duration (Figure 6F, upper panel). When the cells were incubated with dofetilide for 5 hours, the action potential abnormalities appeared more severe (Figure 6F, lower panel).
Chronic exposure to dofetilide alters Nav1.5 channel biophysical properties (Figure 7)
Figure 7.
Effects of 5 hours dofetilide exposure in CHO cells on the voltage-dependence of sodium current inactivation. Panels A and B show examples of tracings recorded using the protocol shown to determine the voltage-dependence of inactivation. In control, inactivation is near-complete by −70 mV, whereas after exposure to dofetilide, currents are not only larger (see also Supplemental Figure 3A–C) but are also incompletely inactivated at −70 mV. Panel C shows that dofetilide produced a striking positive shift in the voltage-dependence of channel inactivation, without a change in the voltage-dependence of activation. As a result, dofetilide increased the overlap between activation and inactivation (dotted box, panel C; shown at expanded scale in panel D). E. A slow voltage ramp protocol shows the peak voltage at which increased window current is observed coincides with the overlap shown in panels C and D.
In the mouse, human iPSC-CM and CHO cell experiments with chronic dofetilide exposure, we noted not only increased INa-L, but also increased peak current (Supplemental Figures 4A–C). The increased peak current was also observed with d-sotalol and E-4031 (Supplemental Figure 4D). Thus, one possibility to further explain the dofetilide effect is increased transcriptional activity with resultant increased Nav1.5 protein. However, Western blots comparing Nav1.5 abundance in CHO cells grown for 48 hours after transfection in the presence or absence of dofetilide (1 µM) showed no difference (n=6 each; Supplemental Figure 4E).
These data suggest that altered gating may contribute to the increases in current seen with chronic exposure to these agents. Figures 7A and 7B show that chronic dofetilide not only increased peak current, but also elicited a dramatic positive shift in the voltage-dependence of inactivation (Figure 7C and Figure 7D); as a result, the region of overlap between steady state activation and inactivation (the “window”; Figure7C and 7D) was enhanced. Further, slow depolarizing ramps also demonstrated the enhanced inward current, maximal at ~−40mV (Figure 7E). The reversal potential for the augmented current, measured with a ramp (Figure 7E), is more positive than when measured conventionally with a step (Supplemental Figure 4C); such a shift has been reported with LQT3 mutant channels that also demonstrate a positive shift in inactivation and augmented INa-L.28 Supplemental Figure 5 shows that chronic dofetilide also accelerated recovery from inactivation (panels A–C): the time constant for recovery from inactivation was 11.9±1.2 msec in untreated cells compared to 8.1±0.7 msec with dofetilide (P=0.0124). In addition, chronic dofetilide increased both the fast and slow time constants of sodium channel inactivation (panel D). These findings support the idea that a “window current” contributes to the persistent current that we have termed INa-L here. ATX-II causes a similar shift in inactivation.17,18
Differing effects of dofetilide and moxifloxacin on Akt phosphorylation (Figure 8)
Figure 8.
Differing effects of dofetilide and moxifloxacin on Akt phosphorylation. A and B. Akt phosphorylation was significantly inhibited by dofetilide (Dof, at 3 and 10 µM, p<0.05), but not by moxifloxacin (Moxi, 100 µM), compared with the positive control PI3K inhibitor LY294002 (20 µM) in CHO cells transfected with SCN5A. Paired t-tests were used for data comparisons. A p<0.025 was considered statistically significant (adjusted for 2 tests). C and D. Chronic (5 hours) LY294002 (20 µM) enhanced late current in SCN5A-transfected CHO cells.
The effect of dofetilide to increase INa-L was reversed by including PIP3 in the pipette. This finding suggests that dofetilide interacts at an as-yet-unidentified point with PI3K signaling. As a further test of this hypothesis, we examined the effect of dofetilide exposure to inhibit phosphorylation of the PI3K target Akt, compared with the PI3K inhibitor LY294002. Figure8A and 8B show partial inhibition of Akt phosphorylation by dofetilide (3 µM, n=7, p=0.018) and no effect of moxifloxacin (100 µM), the IKr blocker that did not enhance INa-L or generate action potential prolongation with long-term exposure in mouse myocytes. This effect was reproducible and seen in HEK cells stably transfected with SCN5A, CHO cells transiently transfected with SCN5A (Figure 8B), and iPSC-CMs. Interestingly, INa-L was also enhanced with exposure of SCN5A-transfected CHO cells to LY294002 (20 µM) for 48 hours (Figure 8C and D), an effect similar to that seen with chronic dofetilide.
Discussion
Current regulatory guidelines for evaluating the potential that a new drug entity causes long QT-related arrhythmogenicity focus on the relationship among IKr block, QT prolongation, and afterdepolarizations.1,29 This evaluation typically includes assessment of a new drug entity’s IKr blocking properties in heterologous expression systems such as CHO cells as well as a “thorough QT study”, in which the QT prolonging effects of low and high drug concentrations are compared to those seen with placebo and with the positive control moxifloxacin in human subjects. The data we present here demonstrate that even drugs thought to be highly specific IKr blockers can be arrhythmogenic via a separate, time-dependent pathway, and that this effect is not seen across all IKr blockers. These findings have important implications discussed below for evaluation of the arrhythmogenic potential of new drug entities and may contribute to variability in an individual subject’s susceptibility to diLQTS.
Dofetilide is one of a family of methanesulfonanilides that include sotalol, E-4031 and almokalant (H23409) that are potent IKr blockers, and devoid of other significant electropharmacologic actions. A structural basis for high potency block of the IKr channel (Kv11.1) encoded by KCNH2 (HERG) by these agents has been described,30 and these drugs have a relatively high propensity to cause TdP. For example, in early clinical trials, the incidence with dofetilide was 3.3% in patients with atrial fibrillation (AF);31 as a result, high dosages are not used, drug dosing is guided by creatinine clearance, and extensive monitoring of the QT interval is undertaken during initiation of therapy. Almokalant caused TdP in one of the first humans exposed32 and was not further developed.
While data from prospective clinical trials are not available, the frequency of serious ventricular arrhythmias such as TdP with other drugs seems much less than 1%: highest with haloperidol and thioridazine,25 occasionally with erythromycin at high concentrations, very rarely with moxifloxacin, and virtually never with verapamil (which has in fact been used to treat TdP). The reason for this variation has never been well-established. One obvious difference is that dofetilide and structural analogs, including racemic sotalol, are generally prescribed in patients with heart disease and most often in those with AF with or without heart failure; by contrast, the “thorough QT study” is generally conducted in normal volunteers.33 We and others have shown that the period following conversion of AF to sinus rhythm carries increased risk for TdP.34 Observations such as these suggest that abnormal cell signaling in heart failure,35 AF, or other settings in which TdP susceptibility is increased causes altered ion channel expression or function (collectively termed remodeling) which then results in action potential and QT interval prolongation. Given that repolarization is a complex process reflecting the activity of multiple inward and outward ionic currents, such remodeling is thought to decrease the “buffering capacity” or “reserve” of action potential control,36,37 and thus increase the likelihood of exaggerated action potential prolongation, and associated arrhythmias, when key components such as IKr or INa-L are perturbed.
PI3K and its downstream effector Akt are critical determinants of heart size38,39 and modulators of hypertrophy.40 Akt activation is thought to modulate the heart failure phenotype though multiple mechanisms, including effects on angiogenesis, apoptosis and other forms of cell death, calcium cycling, and skeletal muscle adaption;41 long term Akt activation is thought to promote heart failure. A role for reduced PI3K activation in modulating susceptibility to AF has also been reported in mice.42 It is thus intriguing to speculate that the relationship between AF or heart failure and perturbed repolarization and arrhythmia susceptibility may be mediated in part by variable pathophysiologic or pharmacologic PI3K inhibition. Our demonstration here that dofetilide but not moxifloxacin inhibits Akt phosphorylation is consistent with the hypothesis that dofetilide interacts directly with the PI3K pathway through mechanisms that remain to be precisely defined to effect the increase in INa-L we have documented here. The finding that pipette PIP3 had no effect on INa-L stimulated by ATX-II lends further support to the idea that the drug-induced increase we report here is mediated by an effect in this pathway.
Chronic dofetilide exposure generated multiple derangements in sodium channel biophysics: a positive shift in channel inactivation, slowed inactivation, and accelerated recovery from inactivation. Taken together, these can account for both the increase in peak and late sodium current we have reported here.43 Further work will be required to elucidate the specific target of the interaction between dofetilide and the PI3K pathway, the extent to which variability in a PI3K effect accounts for variability in TdP liability across drugs, and the extent to which abnormal suppression of this pathway in disease may increase arrhythmogenic drug effects.
It has been argued that the extent of acute IKr block is imperfect at best as a predictor of the effects of a drug in a human subject.44,45 Proposed reasons for this potential disconnect include a time-dependent effect on biosynthesis of the Kv11.1 channel that underlies IKr or on cell surface trafficking,46,47 or failure of in vitro testing to consider other ion channel actions such as L-type calcium channel block48 or increased INa-L.7,49 We show here that even drugs with previously-accepted IKr specificity can exert surprisingly large effects on INa-L, and that this effect is directly arrhythmogenic. However, not all IKr blockers modulate INa-L, and this diversity of effects, in turn, may contribute to the apparent differences in TdP frequency across culprit drugs. The observation that dofetilide, E-4031, and d-sotalol all increased INa-L may reflect their structural similarities as methanesulfonanilides discussed above, but compounds lacking this structure, such as thioridazine, erythromycin or haloperidol, also increase INa-L.
The major implication of the work for the drug development community has to be that relying on an assay that assesses acute block of IKr in a heterologous expression system (or even in an animal or human myocyte) is unlikely to provide a comprehensive assessment of a candidate molecule’s arrhythmogenic potential. This conclusion does not argue against the use of early in vitro studies but rather suggests that these need to look beyond acute effects on IKr. Further, new drug evaluations will need to continue to examine drug effects over time in systems that include all components of normal and perhaps abnormal repolarization, such as hiPSC-derived cardiomyocytes and direct evaluations in human subjects.
Supplementary Material
Acknowledgements
We thank Wei Zhang, Lynn Hall, Laura Short, and Melissa Ryan for assistance with mouse management, myocyte isolation, and conduct of these experiments. Al George provided the stably transfected SCN5A cell lines.
Funding Sources: This work was supported by HL049989, HL104040, HL071670 and HL088635.
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Fenichel RR, Malik M, Antzelevitch C, Sanguinetti M, Roden DM, Priori SG, Ruskin JN, Lipicky RJ, Cantilena LR. Drug-induced torsades de pointes and implications for drug development. J Cardiovasc Electrophysiol. 2004;15:475–495. doi: 10.1046/j.1540-8167.2004.03534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roden DM. Drug-induced prolongation of the QT Interval. New Engl J Med. 2004;350:1013–1022. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- 3.Bloomfield DM, Kost JT, Ghosh K, Hreniuk D, Hickey LA, Guitierrez MJ, Gottesdiener K, Wagner JA. The effect of moxifloxacin on QTc and implications for the design of thorough QT studies. Clin.Pharmacol Ther. 2008;84:475–480. doi: 10.1038/clpt.2008.33. [DOI] [PubMed] [Google Scholar]
- 4.Dale KM, Lertsburapa K, Kluger J, White CM. Moxifloxacin and torsade de pointes. Ann Pharmacother. 2007;41:336–340. doi: 10.1345/aph.1H474. [DOI] [PubMed] [Google Scholar]
- 5.Kim TD, le Coutre P, Schwarz M, Grille P, Levitin M, Fateh-Moghadam S, Giles FJ, Dörken B, Haverkamp W, Köhncke C. Clinical cardiac safety profile of nilotinib. Haematologica. 2012;97:883–889. doi: 10.3324/haematol.2011.058776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kantarjian H, Giles F, Wunderle L, Bhalla K, O'Brien S, Wassmann B, Tanaka C, Manley P, Rae P, Mietlowski W, Bochinski K, Hochhaus A, Griffin JD, Hoelzer D, Albitar M, Dugan M, Cortes J, Alland L, Ottmann OG. Nilotinib in Imatinib-Resistant CML and Philadelphia Chromosome–Positive ALL. New Engl J Med. 2006;354:2542–2551. doi: 10.1056/NEJMoa055104. [DOI] [PubMed] [Google Scholar]
- 7.Lu Z, Wu CY, Jiang YP, Ballou LM, Clausen C, Cohen IS, Lin RZ. Suppression of Phosphoinositide 3-Kinase Signaling and Alteration of Multiple Ion Currents in Drug-Induced Long QT Syndrome. Sci Transl Med. 2012;4:131ra150. doi: 10.1126/scitranslmed.3003623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mounsey JP, DiMarco JP. Dofetilide. Circulation. 2000;102:2665–2670. doi: 10.1161/01.cir.102.21.2665. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Feng ZP, Kondo CS, Sheldon RS, Duff HJ. Developmental changes in the delayed rectifier K + channels in mouse heart. Circ Res. 1996;79:79–85. doi: 10.1161/01.res.79.1.79. [DOI] [PubMed] [Google Scholar]
- 10.Liu K, Yang T, Viswanathan PC, Roden DM. New mechanism contributing to drug-induced arrhythmia: rescue of a misprocessed LQT3 mutant. Circulation. 2005;112:3239–3246. doi: 10.1161/CIRCULATIONAHA.105.564008. [DOI] [PubMed] [Google Scholar]
- 11.Lowe JS, Stroud DM, Yang T, Hall L, Atack TC, Roden DM. Increased late sodium current contributes to long QT-related arrhythmia susceptibility in female mice. Cardiovasc Res. 2012;95:300–307. doi: 10.1093/cvr/cvs160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang T, Atack TC, Stroud DM, Zhang W, Hall L, Roden DM. Blocking Scn10a Channels in Heart Reduces Late Sodium Current and Is Antiarrhythmic. Circ Res. 2012;111:322–332. doi: 10.1161/CIRCRESAHA.112.265173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watanabe H, Yang T, Stroud DM, Lowe JS, Harris L, Atack TC, Wang DW, Hipkens SB, Leake B, Hall L, Kupershmidt S, Chopra N, Magnuson MA, Tanabe N, Knollmann BC, George AL, Jr, Roden DM. Striking In vivo phenotype of a disease-associated human SCN5A mutation producing minimal changes in vitro. Circulation. 2011;124:1001–1011. doi: 10.1161/CIRCULATIONAHA.110.987248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu J, Chau KF, Vodyanik MA, Jiang J, Jiang Y. Efficient feeder-free episomal reprogramming with small molecules. PloS one. 2011;6:e17557. doi: 10.1371/journal.pone.0017557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, Klos M, Wilson GF, Herman AM, Lian X, Raval KK, Barron MR, Hou L, Soerens AG, Yu J, Palecek SP, Lyons GE, Thomson JA, Herron TJ, Jalife J, Kamp TJ. Extracellular Matrix Promotes Highly Efficient Cardiac Differentiation of Human Pluripotent Stem Cells: The Matrix Sandwich Method. Circ Res. 2012;111:1125–1136. doi: 10.1161/CIRCRESAHA.112.273144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsushita Y, Ohya S, Suzuki Y, Itoda H, Kimura T, Yamamura H, Imaizumi Y. Inhibition of Kv1.3 potassium current by phosphoinositides and stromal-derived factor-1α in Jurkat T cells. Am J Physiol Cell Physiol. 2009;296:C1079–C1085. doi: 10.1152/ajpcell.00668.2008. [DOI] [PubMed] [Google Scholar]
- 17.Mantegazza M, Franceschetti S, Avanzini G. Anemone toxin (ATX II)-induced increase in persistent sodium current: effects on the firing properties of rat neocortical pyramidal neurones. J Physiol. 1998;507(Pt 1):105–116. doi: 10.1111/j.1469-7793.1998.105bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macianskiene R, Bito V, Raeymaekers L, Brandts B, Sipido KR, Mubagwa K. Action potential changes associated with a slowed inactivation of cardiac voltage-gated sodium channels by KB130015. Br J Pharmacol. 2003;139:1469–1479. doi: 10.1038/sj.bjp.0705379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang S, Zhou Z, Gong Q, Makielski JC, January CT. Mechanism of block and identification of the verapamil binding domain to HERG potassium channels. Circ.Res. 1999;84:989–998. doi: 10.1161/01.res.84.9.989. [DOI] [PubMed] [Google Scholar]
- 20.Kramer J, Obejero-Paz CA, Myatt G, Kuryshev YA, Bruening-Wright A, Verducci JS, Brown AM. MICE Models: Superior to the HERG Model in Predicting Torsade de Pointes. Sci. Rep. 2013;3 doi: 10.1038/srep02100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirsch GE, Trepakova ES, Brimecombe JC, Sidach SS, Erickson HD, Kochan MC, Shyjka LM, Lacerda AE, Brown AM. Variability in the measurement of hERG potassium channel inhibition: effects of temperature and stimulus pattern. J Pharmacol Toxicol Methods. 2004;50:93–101. doi: 10.1016/j.vascn.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Daleau P, Lessard E, Groleau MF, Turgeon J. Erythromycin blocks the rapid component of the delayed rectifier potassium current and lengthens repolarization of guinea pig ventricular myocytes. Circulation. 1995;91:3010–3016. doi: 10.1161/01.cir.91.12.3010. [DOI] [PubMed] [Google Scholar]
- 23.Katchman AN, Koerner J, Tosaka T, Woosley RL, Ebert SN. Comparative Evaluation of HERG Currents and QT Intervals following Challenge with Suspected Torsadogenic and Nontorsadogenic Drugs. J Pharmacol Exp Ther. 2006;316:1098–1106. doi: 10.1124/jpet.105.093393. [DOI] [PubMed] [Google Scholar]
- 24.Drolet B, Vincent F, Rail J, Chahine M, Deschenes D, Nadeau S, Khalifa M, Hamelin BA, Turgeon J. Thioridazine lengthens repolarization of cardiac ventricular myocytes by blocking the delayed rectifier potassium current. J Pharmacol Exp Ther. 1999;288:1261–1268. [PubMed] [Google Scholar]
- 25.Glassman AH, Bigger JT., Jr Antipsychotic drugs: prolonged QTc interval, torsade de pointes, and sudden death. Am J Psychiatry. 2001;158:1774–1782. doi: 10.1176/appi.ajp.158.11.1774. [DOI] [PubMed] [Google Scholar]
- 26.Liang P, Lan F, Lee AS, Gong T, Sanchez-Freire V, Wang Y, Diecke S, Sallam K, Knowles JW, Wang PJ, Nguyen PK, Bers DM, Robbins RC, Wu JC. Drug Screening Using a Library of Human Induced Pluripotent Stem Cell–Derived Cardiomyocytes Reveals Disease-Specific Patterns of Cardiotoxicity. Circulation. 2013;127:1677–1691. doi: 10.1161/CIRCULATIONAHA.113.001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jonsson MKB, Vos MA, Mirams GR, Duker G, Sartipy P, de Boer TP, van Veen TAB. Application of human stem cell-derived cardiomyocytes in safety pharmacology requires caution beyond hERG. J Mol Cell Cardiol. 2012;52:998–1008. doi: 10.1016/j.yjmcc.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Song W, Xiao Y, Chen H, Ashpole NM, Piekarz AD, Ma P, Hudmon A, Cummins TR, Shou W. The human Nav1.5 F1486 deletion associated with long QT syndrome leads to impaired sodium channel inactivation and reduced lidocaine sensitivity. J Physiol. 2012;590:5123–5139. doi: 10.1113/jphysiol.2012.235374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fermini B, Fossa AA. The impact of drug-induced QT interval prolongation on drug discovery and development. Nat Rev Drug Discov. 2003;2:439–447. doi: 10.1038/nrd1108. [DOI] [PubMed] [Google Scholar]
- 30.Mitcheson JS, Chen J, Lin M, Culberson C, Sanguinetti MC. A structural basis for drug-induced long QT syndrome. Proc Natl Acad Sci U S A. 2000;97:12329–12333. doi: 10.1073/pnas.210244497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torp-Pedersen C, Moller M, Bloch-Thomsen PE, Kober L, Sandoe E, Egstrup K, Agner E, Carlsen J, Videbaek J, Marchant B, Camm AJ. Dofetilide in patients with congestive heart failure and left ventricular dysfunction. Danish Investigations of Arrhythmia and Mortality on Dofetilide Study Group. New Engl J Med. 1999;341:857–865. doi: 10.1056/NEJM199909163411201. [DOI] [PubMed] [Google Scholar]
- 32.Wiesfeld AC, Crijns HJ, Bergstrand RH, Almgren O, Hillege HL, Lie KI. Torsades de pointes with Almokalant, a new class III antiarrhythmic drug. Am Heart J. 1993;126:1008–1011. doi: 10.1016/0002-8703(93)90726-p. [DOI] [PubMed] [Google Scholar]
- 33.Shah R. Drugs, QTc Interval Prolongation and Final ICH E14 Guideline. Drug-Safety. 2005;28:1009–1028. doi: 10.2165/00002018-200528110-00003. [DOI] [PubMed] [Google Scholar]
- 34.Choy AMJ, Darbar D, Dell'Orto S, Roden DM. Increased sensitivity to QT prolonging drug therapy immediately after cardioversion to sinus rhythm. J Am Coll Cardiol. 1999;34:396–401. doi: 10.1016/s0735-1097(99)00226-0. [DOI] [PubMed] [Google Scholar]
- 35.Kaab S, Duc J, Ashen D, Näbauer M, Beuckelmann DJ, Dixon J, McKinnon D, Tomaselli GF. Quantitative analysis of K channel mRNA expression in normal and failing human ventricle reveals the molecular identity of Ito1. Circulation. 1996;94:I-592. [Google Scholar]
- 36.Roden DM. Long QT syndrome: reduced repolarization reserve and the genetic link. J Intern Med. 2006;259:59–69. doi: 10.1111/j.1365-2796.2005.01589.x. [DOI] [PubMed] [Google Scholar]
- 37.Roden DM. Repolarization reserve: a moving target. Circulation. 2008;118:981–982. doi: 10.1161/CIRCULATIONAHA.108.798918. [DOI] [PubMed] [Google Scholar]
- 38.Shioi T, Kang PM, Douglas PS, Hampe J, Yballe CM, Lawitts J, Cantley LC, Izumo S. The conserved phosphoinositide 3-kinase pathway determines heart size in mice. EMBO J. 2000;19:2537–2548. doi: 10.1093/emboj/19.11.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shioi T, McMullen JR, Kang PM, Douglas PS, Obata T, Franke TF, Cantley LC, Izumo S. Akt/Protein Kinase B Promotes Organ Growth in Transgenic Mice. Mol Cell Biol. 2002;22:2799–2809. doi: 10.1128/MCB.22.8.2799-2809.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Kang PM, Izumo S. Phosphoinositide 3-kinase(p110α) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci U S A. 2003;100:12355–12360. doi: 10.1073/pnas.1934654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaanine AH, Hajjar RJ. AKT signalling in the failing heart. Eur J Heart Fail. 2011;13:825–829. doi: 10.1093/eurjhf/hfr080. Epub 2011 Jun 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pretorius L, Du X-J, Woodcock EA, Kiriazis H, Lin RCY, Marasco S, Medcalf RL, Ming Z, Head GA, Tan JW, Cemerlang N, Sadoshima J, Shioi T, Izumo S, Lukoshkova EV, Dart AM, Jennings GL, McMullen JR. Reduced Phosphoinositide 3-Kinase (p110α) Activation Increases the Susceptibility to Atrial Fibrillation. Am J Pathol. 2009;175:998–1009. doi: 10.2353/ajpath.2009.090126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shryock JC, Song Y, Rajamani S, Antzelevitch C, Belardinelli L. The arrhythmogenic consequences of increasing late INa in the cardiomyocyte. Cardiovasc Res. 2013;99:600–611. doi: 10.1093/cvr/cvt145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoffmann P, Warner B. Are hERG channel inhibition and QT interval prolongation all there is in drug-induced torsadogenesis? A review of emerging trends. J Pharmacol Toxicol Methods. 2006;53:87–105. doi: 10.1016/j.vascn.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 45.Lu HR, Vlaminckx E, Hermans AN, Rohrbacher J, Van Ammel K, Towart R, Pugsley M, Gallacher DJ. Predicting drug-induced changes in QT interval and arrhythmias: QT-shortening drugs point to gaps in the ICHS7B Guidelines. Br J Pharmacol. 2008;154:1427–1438. doi: 10.1038/bjp.2008.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ficker E, Kuryshev YA, Dennis AT, Obejero-Paz C, Wang L, Hawryluk P, Wible BA, Brown AM. Mechanisms of arsenic-induced prolongation of cardiac repolarization. Mol Pharmacol. 2004;66:33–44. doi: 10.1124/mol.66.1.33. [DOI] [PubMed] [Google Scholar]
- 47.Kuryshev YA, Ficker E, Wang L, Hawryluk P, Dennis AT, Wible BA, Brown AM, Kang J, Chen XL, Sawamura K, Reynolds W, Rampe D. Pentamidine-induced long QT syndrome and block of hERG trafficking. J Pharmacol Exp Ther. 2005;312:316–323. doi: 10.1124/jpet.104.073692. [DOI] [PubMed] [Google Scholar]
- 48.Kuryshev YA, Wang L, Wible BA, Wan X, Ficker E. Antimony-Based Antileishmanial Compounds Prolong the Cardiac Action Potential by an Increase in Cardiac Calcium Currents. Mol Pharmacol. 2006;69:1216–1225. doi: 10.1124/mol.105.019281. [DOI] [PubMed] [Google Scholar]
- 49.Lacerda AE, Kuryshev YA, Chen Y, Renganathan M, Eng H, Danthi SJ, Kramer JW, Yang T, Brown AM. Alfuzosin delays cardiac repolarization by a novel mechanism. J Pharmacol Exp Ther. 2008;324:427–433. doi: 10.1124/jpet.107.128405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.