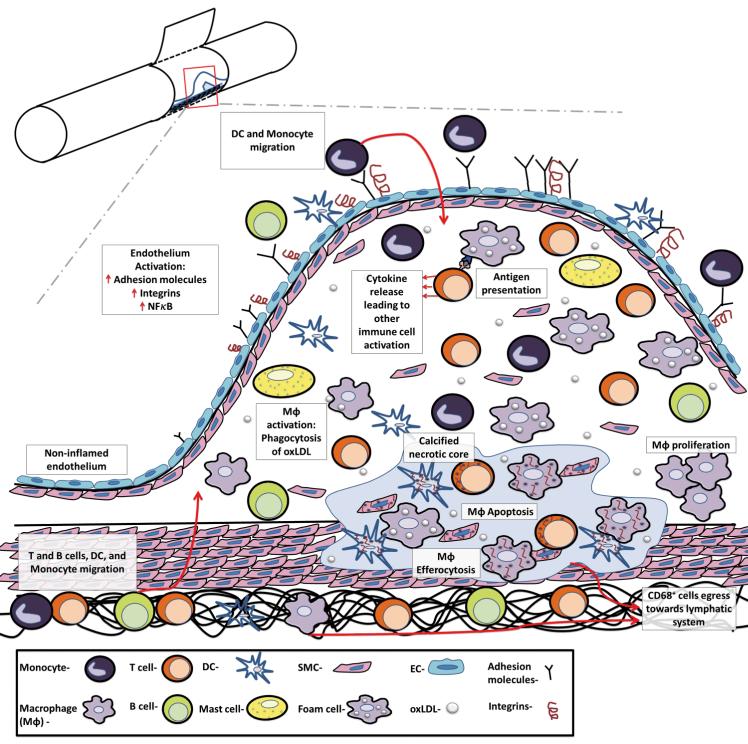
Figure 1.
The major steps in the development and progression of atherosclerosis. Atherosclerosis is a progressive, multifactorial inflammatory disease that encompasses endothelial activation, leukocyte recruitment, MΦ activation and proliferation, and antigen presentation to T cells and ultimately results in elevated pro-inflammatory cytokine production, inflammation, and necrosis. Atherosclerosis is initiated by the deposition, retention, and modification of LDL in the intima, resulting in NF-κB–dependent activation of the endothelium, the expression of adhesion molecules, chemokines, and the recruitment of Ly6Chi monocytes and pre-DCs to the intimal layer. Ly6Chi monocytes may subsequently differentiate to MΦ or possibly DCs. MΦs are the prominent cell type within the arterial wall, and several arterial macrophage subsets (M1, M2, M4, and Mox MΦ, Figure 2) have been observed. Dendric cells and DC subsets (Flt3-dependent DCs, Flt3-independent DCs, and pDCs, not shown), while far less abundant, are similarly present. MΦs and DCs scavenge for modified lipoproteins (or other potential self-antigens) within the arterial lumen by scavenger receptors and Toll-like receptors, and may present antigens to T cells to activate intra-plaque T helper (Th) 1 and Th17 cells, and thus to further support leukocyte recruitment to the nascent plaque. Once activated, arterial MΦs become either pro-inflammatory proteolytic M1 MΦs, releasing IL-12, IL-1β, and TNF-α, or less inflammatory M2 MΦs, releasing IL-10 and TGF-β. DC subsets also support atherosclerotic low grade inflammation by the release of type 1 interferons, and activation/modulation of T cell responses. B cells, which are also present in atherosclerotic plaques, may support or suppress inflammation through the production of antibodies and cytokines. As MΦs start to proliferate and as a result of persistent foam cells formation and reduced efferocytosis, the nascent plaque becomes an advanced atherosclerotic lesion, resulting in the formation of a calcified necrotic core. Upon the resolution of hypercholersterolemia, CD68+ cell egression from the plaque through adventitial lymphatic vessels and efficient efferocytosis might result in regression of the lesion.