Fig. (2).
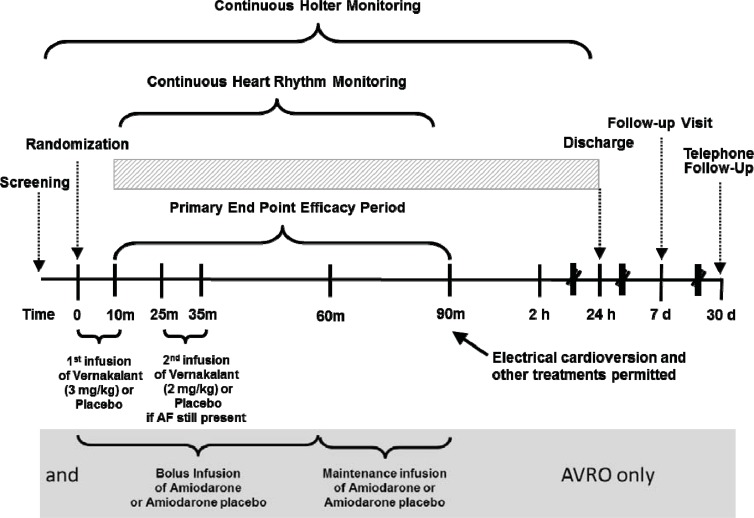
Schematic of the clinical trials for the development of vernakalant. All trial used sinus rhythm at 90 minutes after the start of the vernakalant infusion as the primary endpoint. In CRAFT the infusion doses were reversed. In CRAFT, ACT I, II and III patients were randomised to blinded vernakalant or placebo. In AVRO patients received either amiodarone or vernakalant (or appropriate placebos) in a in a double-blind, double-dummy design. ACT IV was not controlled, but contributed to the safety database of vernakalant.