FIGURE 6.
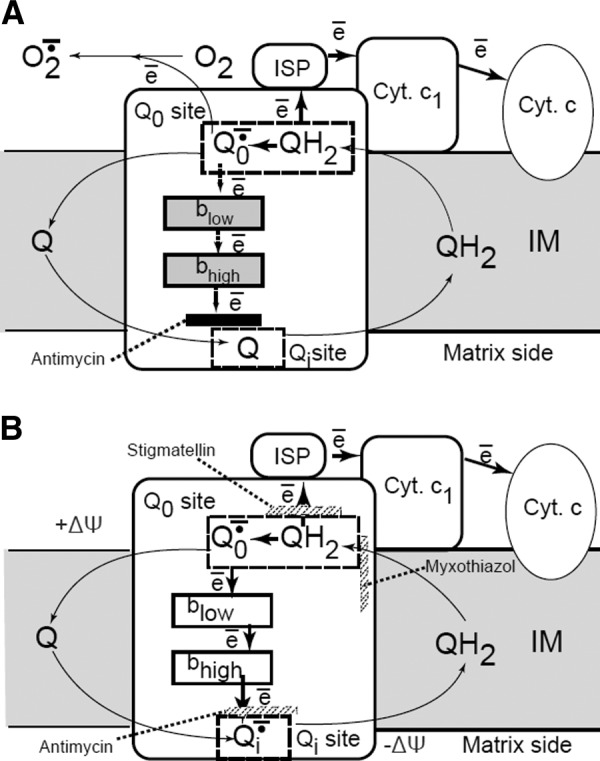
Q-cycle model. The mechanism of superoxide formation in complex III (bcl1 complex). The reaction starts from the oxidation of the CoQ quinol (QH2) in a bifurcated electron transfer reaction at the Qo site of the complex III. The first electron is transferred to a high reduction potential chain consisting of the iron-sulfur protein (ISP, aka Rieske protein), cytochrome c1 (cyt c1), cytochrome c (cyt c), and further to cytochrome c oxidase (not shown). The remaining semiquinone (Qo−·) is unstable. It donates the second electron to the low reduction potential chain consisting of two cytochromes b, cyt bL and cyt bH, which serve as a pathway conducting electrons to the Qi site. There these electrons reduce another CoQ molecule. To provide two electrons required for the complete reduction of CoQ quinone at the Qi-site, the Qo-site oxidizes two QH2 molecules in two successive steps. The first electron at the Qi-site generates a stable semiquinone (Qi−) that is reduced to a quinol (QH2) by the second electron. Most frequently used inhibitors of complex III, stigmatellin and myxothiazol, prevent the transfer of the first electron to ISP and the binding of the quinol at the Qo site correspondingly. [Modified from Starkov and Fiskum (418), with permission from Elsevier.]
