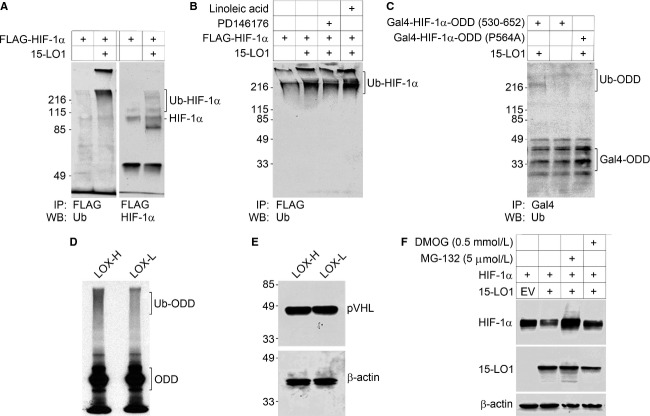
Figure 4.
15-LO1 promotes the ubiquitination and degradation of HIF-1α in normoxia. Both in vivo and in vitro ubiquitination assays were used to elucidate the mechanism of 15-LO1 mediated HIF-1α inhibition. Cells in all experiments were cultured under normoxia (20% O2). A series of in vivo ubiquitination assay were conducted in HEK293 cells (A–C). (A) The effect of 15-LO1 on HIF-1α ubiquitination (Ub-HIF-1α) was detected by immunoprecipitation. (B) The effect of 15-LO1 on HIF-1α ubiquitination in the presence of 15-LO1 inhibitor PD146176 and 15-LO1 substrate linoleic acid. (C) The effect of 15-LO1-induced ubiquitination of the HIF-1α with either wild-type ODD (530-652) domain or ODD domain mutant (P564A). The ubiquitination of HIF-1α polypeptide with wild-type ODD domain was detected as increased Ub-ODD fraction. At the front, fast migrating Gal4-ODD fraction represents un-ubiquitinated ODD. (D) In an in vitro ubiquitination assay, the ubiquitination of radiolabeled HIF-1α ODD polypeptide (530-652) was detected in LOX-H or LOX-L cells. (E) Western blot analysis showing pVHL expression in LOX-H and LOX-L cells (Upper panel), β-actin levels as loading control (Lower panel). (F) A representative Western blotting analysis of HIF-1α expression in HEK293 cells following HIF-1α and 15-LO1 co-transfection in the presence of 26S proteasome inhibitor MG132 and PHD inhibitor DMOG.