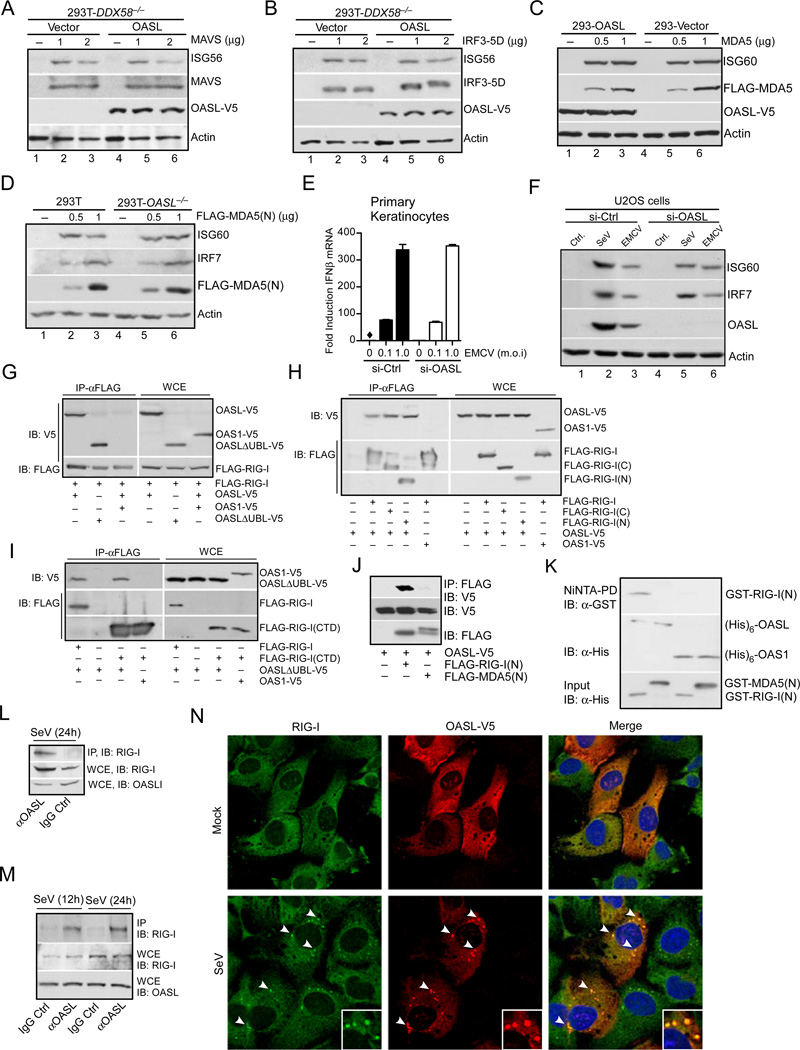
Fig. 6. OASL binds to and specifically enhances RIG-I signaling.
(A–B) OASL does not affect RLR signaling downstream of MAVS. 293T-DDX58−/− cells stably expressing OASL or vector control were transfected with indicated plasmids to express either MAVS (A) or constitutively active IRF3-5D (B) for 24 h followed by immunoblot analysis. (C) OASL does not enhance MDA5 signaling. Cells were transfected either with vector or two different concentrations of FLAG-MDA5 plasmid for 24 h followed by IB. (D) Loss of OASL expression does not affect MDA5 signaling. Cells were transfected for 24 h with indicated amounts of MDA5(N) plasmid and analyzed by IB. (E) OASL silencing does not affect IFNβ induction stimulated by EMCV infection in keratinocytes. Primary human keratinocytes were transfected with 50 nM OASL siRNA and control siRNA for 48 h, followed by EMCV infection for another 24 h. The OASL silencing (Suppl. Fig. S1E), IFNβ production (E) and EMCV replication (Suppl. Fig. S1E) were analyzed by specific qRT-PCR. (F) OASL does not affect the gene ISG60 and IRF7 induction triggered by a physiological MDA5 agonist. 293T cells were infected either with EMCV, VSV and SeV or mock infected (Ctrl.) and extracted for total RNA as described before (Jiang et al., 2012). U2OS cells were first transfected with control or OASL siRNA as described in Fig. 1 for 48 h followed by transfection with 1mg of total RNA prepared form infected cells as indicated. Cell lysates were analyzed by IB. (G) RIG-I interacts with OASL. 293T cells were co-transfected for 24 h with FLAG-RIG-I and OASL-V5, OASL-ΔUBL-V5 or OAS1-V5 plasmids, immunoprecipitated (IP) with FLAG antibody, followed by IB with V5 antibody. Whole cell extracts (WCE) from transfected cells were similarly analyzed for expression levels. (H) Both N-terminal CARD and C-terminal Helicase-CTD domains of RIG-I interact with OASL. 293T cells were either individually transfected with OASL-V5 or co-transfected as before with indicated plasmids followed by IP analysis. (I) OAS domain of OASL interacts with RIG-I CTD domain. 293T cells were transfected as indicated, followed by similar IP analysis. (J) RIG-I CARD domain (RIG-I(N)) specifically interacts with OASL, while MDA5 CARD domain (MDA5(N)) does not. 293T cells were transfected as indicated followed by IP analysis. (K) Specific in vitro interaction of RIG-I(N) with OASL. GST-RIG-I(N) and GST-MDA5(N) proteins purified from bacteria were incubated on ice as indicated with (His)6-OASL or with (His)6-OAS1 purified from insect cells followed by Ni-NTA pull down and IB. (L) Interaction of endogenous OASL and RIG-I. HEK293 cells were infected with SeV (100 HAU/ml) for 24 h. Cell lysates were immunoprecipitated either with OASL antibody or control IgG followed by IB with RIG-I antibody. Control WCE was analyzed to show expression. (M) Interaction of endogenous RIG-I and OASL in primary human fibroblsts. Human primary foreskin fibroblasts (HFF) were stimulated with SeV (160 HAU/ml) for 12 and 24 h respectively, followed by IP analysis as indicated. (N) OASL co-localizes with RIG-I in virus infected cells. HT1080-OASL cells were either mock infected or infected with 100 HAU/ml SeV for 8 h immuno-stained with RIG-I and V5 antibodies followed by confocal microscopy. The arrows denote the puntae formation, and insets show magnified puntaes.