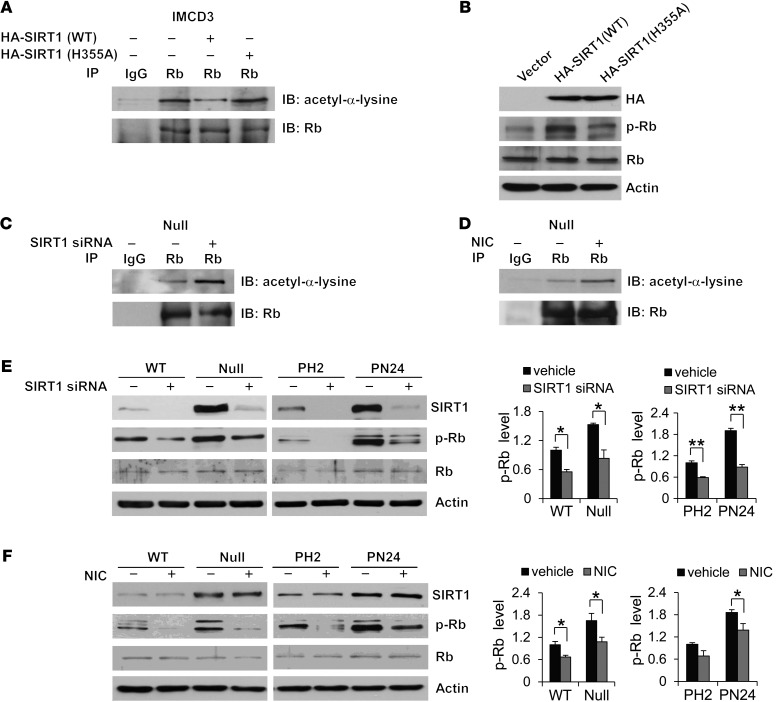
Figure 9. Interaction of SIRT1 with Rb mediates Rb deacetylation and phosphorylation.
(A and B) Overexpression of WT SIRT1, but not deacetylase catalytically inactive mutant SIRT1 H355A, (A) decreased the level of acetylated Rb, as examined with anti–acetyl-α-lysine antibody after Rb was pulled down with anti-Rb antibody, and (B) increased the level of phospho-Rb, as examined by Western blot, in mouse IMCD3 cells transfected with WT SIRT1, mutant SIRT1 H355A, or empty vector for 48 hours. (C and D) Knockdown of SIRT1 with siRNA or inhibition of SIRT1 with nicotinamide increased the acetylation level of Rb in Pkd1-null MEK cells that were (C) transfected with SIRT1 siRNA for 48 hours or (D) treated with 10 mM nicotinamide for 24 hours. Rb acetylation was analyzed as above. (E and F) Knockdown of SIRT1 with siRNA or inhibition of SIRT1 with nicotinamide decreased Rb phosphorylation, but did not affect Rb expression, in WT MEK, Pkd1-null MEK, PH2, and PN24 cells that were (E) transfected with SIRT1 siRNA for 48 hours or (F) treated with 10 mM nicotinamide for 24 hours. *P 0.05; **P 0.01.