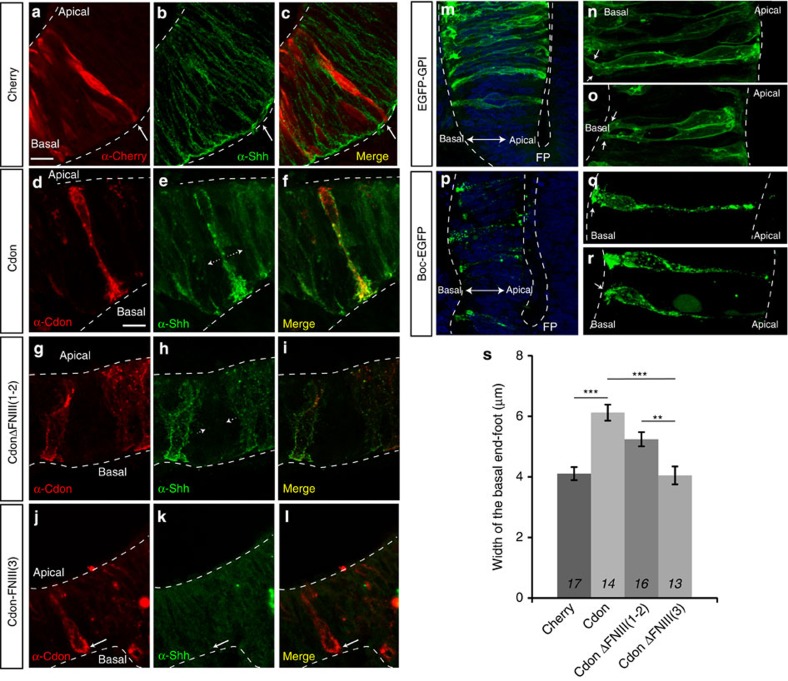
Figure 7. Cdon and Boc promote morphological changes of the neuroepithelial basal side where Shh preferentially accumulates.
(a–r) Confocal analysis of coronal sections at the level of HH14 chicken optic stalk (a–l) and HH10 neural tube (m–r) electroporated at HH8 with a construct carrying mCherry (a–c) Cdon (d–f), CdonΔFnIII(1-2) (g–i), CdonΔFnIII(3) (j–l), EGFP-GPI (m–o) or Boc-EGFP (p–r). Sections in (a–l) were immunostained with antibodies against Cherry (a) Cdon (d,g,j) and Shh (b,e,h,k). Images in (n,o) and (q,r) are high magnification views of cells shown in (m,p) respectively. Note how cells electroporated with Boc-EGFP (q,r, arrows), Cdon (d) or CdonΔFnIII(1-2) (g) present an enlarged basal end-foot when compared with EGFP, mCherry or CdonΔFnIII(3) neuroepithelial cells (n,o and a,j arrows). This enlarged end-foot is a preferential site of Shh accumulation (e,f,h,i). Note also the absence of Shh signal in the cells immediately surrounding the Cdon-positive cells (e,h, dotted line arrows). This distribution is not observed n cells expressing mCherry (b,c) or CdonΔFnIII(3) (k,l). (s) Quantification of the basal end-foot width of neuroepithelial cells electroporated with Cdon and its deleted versions. The number of cases analysed for each data set is indicated in the respective column. The numbers of quantified cells are indicated in the graph labels and are as follows: Cherry, n=17; Cdon, n=14; CdonΔFnIII(1-2), n=16 and CdonΔFnIII(3), n=13. Error bars represent s.e.m. (**P<0.01, ***P<0.001; Student’s t-test). There is no statistical difference in the basal end-foot width between Cdon and CdonΔFnIII(1-2) expressing cells (P=0.066, Student’s t-test) or between Cherry and CdonΔFnIII(3) expressing cells (P=0.998; Student’s t-test). Scale bar, 10 μm.