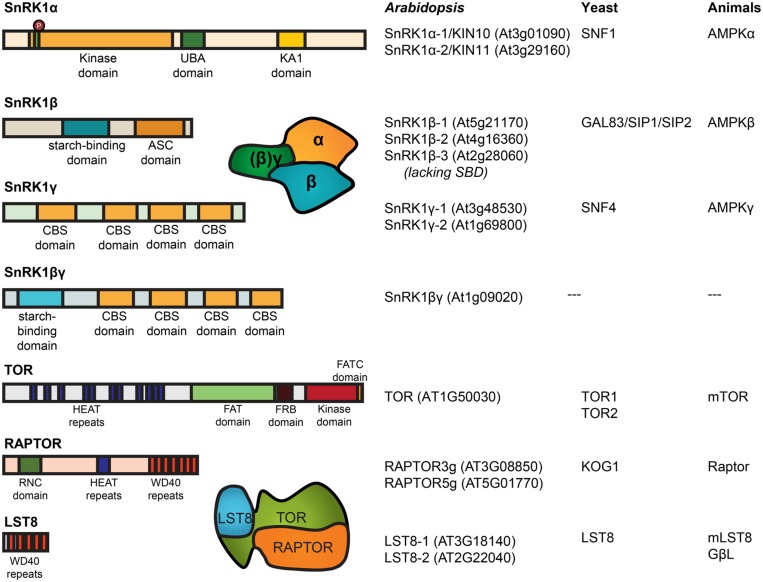
FIGURE 2.
Domain structure and nomenclature of Arabidopsis SnRK1 and TOR subunits. SnRK1 structures include the conserved phosphorylation sites on T-loop of the α-subunit. The α-subunit contains the kinase domain, together with an auto-inhibitory (UBA) domain and a kinase associated (KA1) domain where the interaction with the ß-subunit takes place. The β-subunit (except in SnRK1ß3) contains a starch-binding domain (Ávila-Castañeda et al., 2014) and binds to the γ-subunit at the association with the SNF1 complex (ASC) domain. The plant-specific βγ-subunit might take the place described for the γ-subunit in mammals and yeast, containing multiple cystathionine β-synthase (CBS) domains. TOR contain two FAT domains (FRAP, ATM, and TRAP) probably constituting the active center, a PI3K kinase domain, a FRB domain (FKB12-rapamycin binding) for interaction with the inhibitor FKB12, and a number of HEAT repeats [huntingtin, elongation factor 3 (EF3), protein phosphatase 2A (PP2A), TOR1] for the interaction with RAPTOR. Next to HEAT repeats, RAPTOR contains a RNC domain (raptor N-terminal conserved/putative caspase domain) and a number of WD40 repeat domains (Hay and Sonenberg, 2004). Nomenclature as described before (Robaglia et al., 2012).