Abstract
Cell cycle inhibition has yet to offer a generally effective approach to cancer treatment, but a full evaluation of different combinations of cell cycle inhibitors has yet to be evaluated. Cyclin A2, a core component of the cell cycle, is often aberrantly expressed in cancer where it may impact cell proliferation. In this study, we investigated the role of cyclin A2 in tumorigenesis using a conditional genetic knockout mouse model. Cyclin A2 deletion in oncogene-transformed MEFs suppressed tumor formation in immunocompromised mice. These findings were confirmed in mice with cyclin A2-deficient hepatocytes, where a delay in liver tumor formation was observed. Since cyclin A2 acts in complex with Cdk2 in the cell cycle, we explored a hypothesized role for Cdk2 dysregulation in this effect through conditional deletions of both genes. In oncogene-transformed MEFs lacking both genes, tumor formation was strongly suppressed in a manner associated with decreased proliferation, premature senescence and error-prone recovery from serum deprivation after immortalization. Whereas loss of cyclin A2 led to a compensatory increase in Cdk1 activity, this did not occur with loss of both Cdk2 and cyclin A2. Our work offers a rationale to explore combinations of Cdk1 and Cdk2 inhibitors as a general approach in cancer therapy.
Keywords: knockout mice, Cdk2, cyclin A2, Cdk1, cancer
Introduction
Cyclin A2 binds to and activates its catalytic partners, Cdk2 and Cdk1. Cdk/cyclin A2 complexes phosphorylate proteins like pocket proteins (Rb, p107, p130) and proteins involved in DNA synthesis, thereby driving S phase progression (1-4). In line with its role in regulating S phase and as a typical E2F target gene, cyclin A2 expression is induced upon entry into S phase (1, 5), persists through the S and G2 phase, and is degraded upon entry into mitosis (6, 7). In vitro studies have postulated a role for cyclin A2 in the G2 to M phase transition (2, 8, 9). Germline cyclin A2 knockout mice die at embryonic stage E5.5 indicating an essential role for cyclin A2 in embryonic development (10). The use of conditional knockout mice has revealed that cyclin A2 is essential for cell cycle progression of hematopoietic and embryonic stem cells, but is dispensable for proliferation of mouse embryonic fibroblasts (MEFs) due to the redundant functions of E-type cyclins (11).
Aberrant expression of cyclin A2 has been detected in a variety of cancers and deregulation of cyclin A2 appears to be closely related to chromosomal instability and tumor proliferation (3, 12, 13). Cyclin A2 expression in patient tumors also appears to have prognostic value (14-19) and inhibition of cyclin A2 complexes has been shown to impair proliferation of tumor cell lines (20). Here we developed a conditional knockout mouse model to address the in vivo role of cyclin A2 in cancer.
Methods
Generation of cyclin A2 conditional knockout mice
Mouse genomic DNA harboring the cyclin A2 locus was sub-cloned from the BAC clone RP23-297G4 (ResGen, PKB829) into the pBlight-TK vector. The locus was cloned by ligating two 4.5 kb fragments; primers 5′-CTTCATGGTCTGATGTCACATCGATCGATCGGGATTAGCAAAATTAAT-3′ (PKO484) and 5′-GATATCGATGCCTATGCTCATTCAAGGCATGTGAAATCC-3′ (PKO481) were used to PCR amplify the 5′ fragment, and primers 5′-CAACCACATTTTATCACACTATCGATAGT TTGAAAGTGTGGCTCTT-3′ (PKO485) and 5′-GAAGGTCGACAGCTCATGAAATAGGCC AGAGAGATGGTTC-3′ (PKO483) were used for the 3′ fragment. LoxP recombination sites and a neomycin-selection cassette were introduced flanking the third and fifth coding exons of the mouse cyclin A2 genomic locus using recombineering technique (30). The resulting targeting vector (PKB871; Fig. S1A) was linearized by PvuI digestion and electroporated into ES cells. Following positive and negative selection with Geneticin and ganciclovir, respectively, genomic DNA from surviving ES cell colonies was used to screen for homologous recombination by Southern hybridization (Fig. S1B). ES cell clones (2020, 2023) that were correctly targeted were identified and used for the generation of the cyclin A2 conditional knockout mouse strain. To generate the cyclin A2flox allele, the neomycin cassette was removed by crossing cyclin A2 conditional knockout mice with β-actin–Flpe transgenic mice (31) [strain name: B6.Cg-Tg(ACTFLPe) 9205Dym/J; stock no.: 005703; The Jackson Laboratory]. Cyclin A2flox mice were crossed with β-actin–Cre transgenic mice (32) [strain name: FVB/N-Tg(ACTB-cre)2Mrt/J; stock no.: 003376; The Jackson Laboratory] to obtain cyclin A2WT/null mice that were then intercrossed. Consistent with the embryonic lethality reported previously for cyclin A2 knockout mice (10, 11), no cyclin A2−/− pups were obtained (data not shown).
Isolation of primary mouse embryonic fibroblasts
Isolation of primary mouse embryonic fibroblasts (MEFs) has been described previously (24). Briefly, the head and the visceral organs of E13.5 embryos were removed and the embryonic tissue was finely chopped using a razor blade. Following trypsinization at 37°C for 20 minutes, tissue and cell clumps were dissociated by pipetting and cells were plated in a 10 cm culture dish (passage 0). MEFs were cultured in DMEM (Invitrogen 12701-017) supplemented with 10% fetal calf serum (Invitrogen 26140) and 1% penicillin/streptomycin (Invitrogen 15140-122) at 37°C in a humidified incubator with 5% CO2 and 3% or 21% O2.
Cell culture and FACS analysis
Cells were stained for SA-β-galactosidase to detect senescence (33). Following knockout of cyclin A2 by addition of 4-OHT for 48 hours, MEFs were collected at various passages, seeded in 6 well dishes and washed twice with PBS the next day. Incubation for 5 minutes in fixative solution [2%(wt/vol) formaldehyde/0.2% (wt/vol) glutaraldehyde solution in PBS] was followed by overnight incubation at 37°C in staining solution [20 mM citric acid, 40 mM Na2HPO4 (pH 6.0), 150 mM NaCl, 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 2 mM MgCl2, 1 mg/mL X-Gal (Invitrogen 15520034)]. Blue stained cells were visualized using Olympus CKX41 microscope and images were taken with an Olympus E-330 camera.
For 3T3 and proliferation assays, cyclin A2 knockout was achieved by addition of 4-OHT to MEFs for 48 hours, followed by plating 300,000 MEFs in 10 cm dishes (3T3 assay) or 1,500 cells in five replicates in 96-well plates (proliferation assay). In the 3T3 protocol, cells were trypsinized and counted every 3 days, followed by re-plating of 300,000 cells (26). For proliferation assays, cells were incubated for 4 hours in 150 μl of detection reagent [alamarBlue (BUF012B; AbD Serotec) diluted 1:10 in growth medium]. Proliferation rates were estimated as a measure of metabolic activity by measuring fluorescence at 590 nm every 24 hours. For RO3306 experiments, confluent MEFs were serum starved in growth medium containing 0.1% serum for 72 hours, and cyclin A2 knockout was achieved concurrently by addition of 20ng/ml 4-OHT during the starvation period. MEFs were then plated for proliferation assay in medium containing 10% serum and different concentrations of RO3306 (Calbiochem 217699).
For colony formation assays, cells were infected with the retroviral constructs LXSN-HRas/p53DN (PKB816) or LXSN-H-Ras/Myc (PKB817). 48 hours after infection, 10,000 cells were plated in 10 cm dishes containing growth medium with or without 4-OHT. Colonies were visualized 6-8 days later by fixing cells in ice-cold methanol and staining with 0.02% Giemsa solution (Sigma GS-500). p53 inactivation was confirmed by treating infected cells with 1 μM Adriamycin for 24 hours, and analyzing cell extracts for p53 expression.
Silencing of Cdk2 in primary liver tumor cells was performed by infection of cells with retroviral shRNA construct pMKO.1-shCdk2C (PKB824) kindly provided by Piotr Sicinski (34). This construct has been previously validated (34) and used by us in an earlier study (23).
For FACS analysis of unsynchronized MEFs, cyclin A2 knockout was induced by addition of 20ng/ml 4-OHT (Sigma H7904) for 48 hours. For FACS analysis of synchronized MEFs, confluent MEFs were serum starved in growth medium containing 0.1% serum for 72 hours, and cyclin A2 knockout was achieved concurrently by addition of 20ng/ml 4-OHT during the starvation period. MEFs were released into 10% serum containing medium and collected at various time points. One hour prior to collection of MEFs, cells were labeled with 100μM BrdU (BD Pharmingen 550891) to monitor S phase. Cells were trypsinized, fixed in ice cold 70% ethanol, stained with APC-conjugated anti-BrdU antibodies (BD Pharmingen 623551) and propidium iodide (Sigma 81845). Cell-cycle analysis was performed using a FACS-Calibur flow cytometer (BD Biosciences) and FlowJo 8 software.
Western blot analysis, immunoprecipitation, and kinase assays
Lysates from MEFs were prepared as described previously (23) and used for Western blots or kinase assays. The following antibodies were used in this study: cyclin A2 – Santa Cruz Biotechnology #SC-596, cyclin B1 – Cell Signaling #4135 and #4138, cyclin E1 – eBioscience #14-6714-63, Cdk1 & Cdk2 as described (24), HSP90 – BD Biosciences #610418, p53 – Cell Signaling #2524, p21 – Santa Cruz Biotechnology #SC-6246, p27 – BD Transduction Laboratories #610242, p27 – Invitrogen #71-9600, Erk1/2 – Cell Signaling #4695, Phospho-Erk1/2 – Cell Signaling #9101, Actin – Santa Cruz Biotechnology #SC-1616, c-Myc – Santa Cruz Biotechnology #SC-42. For immuno-precipitation and kinase assays, cross-linked antibodies against Cdk2 and cyclin B1 [as described in (23)], Cdk1 (Santa Cruz #SC-54AC), cyclin A2 (Santa Cruz #SC-751AC), cyclin B1 (Santa Cruz #SC-7393AC), and suc1 agarose beads (Millipore #14-132) were used. Kinase assays with immunoprecipitated proteins using Histone H1 as substrate were performed as described previously (23).
Tumorigenesis in nude mice
Primary MEFs were transformed by infection with retroviral construct LXSN-H-Ras/p53DN (PKB816). Following induction with 4-OHT for 48 hours to achieve cyclin A2 knockout, 1 × 106 MEFs in PBS were injected subcutaneously into the flanks of nude mice. Tumor measurements were taken twice a week until tumor diameters reached 20 mm, upon which mice were sacrificed. For analysis of cyclin A2 deletion in growing tumors, transformed MEFs were injected subcutaneously into the flanks of nude mice. Once tumor volumes reached 60-100 mm3 (7-10 days after injection), Tamoxifen pellets (Innovative Research of America, SE-361 Tamoxifen Free Base, 25mg/pellet, 60 day release) were subcutaneously implanted at a site distal to the tumor. Tumor volumes (V) were calculated using this formula: V= L x W x W/2 (L: length, W: width). All procedures were done in compliance with IACUC guidelines.
Liver tumorigenesis
Sleeping Beauty transposon induced liver tumorigenesis by hydrodynamic tail vein injections was performed as described previously (22, 23). 2-4 month old mice were injected with a plasmid cocktail diluted in lactated Ringer’s solution through their lateral tail vein in less than 10 seconds using 27-gauge needles. The Qiagen EndoFree Plasmid Maxi kit (12362) was used for purification of plasmid DNA. 15 μg of transposase (pGK-SleepingBeauty13) and a total of 30 μg of transposon [pT2-Caggs-NRasV12 and pT2-shRNA p53 (35)] encoding plasmids were injected per mouse. The injection volume corresponded to 10% of mouse body weight (i.e. 2 ml for a 20g mouse, not exceeding 3 ml). Mice were sacrificed when moribund, which was within 3-4 months for control mice (those expressing cyclin A2 in their liver) and 6-8 months for test mice (those with cyclin A2 knockout liver), followed by histological analysis. Ki67 positive cells in liver tumor sections were enumerated using Slidepath Digital Image Hub and TissueIA (Leica Microsystems) image analysis programs. All procedures were done in compliance with IACUC guidelines.
Results and Discussion
Delayed tumorigenesis and impaired tumor cell proliferation upon concurrent loss of Cdk2 and cyclin A2
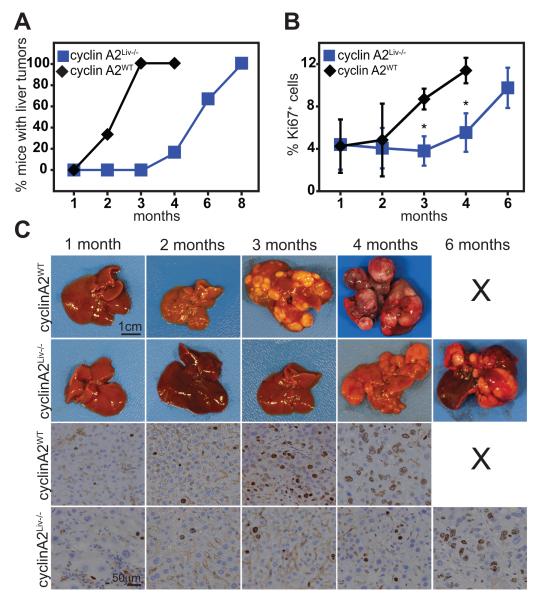
To investigate the roles of cyclin A2 in tumorigenesis in vivo, we generated hepatocyte-specific cyclin A2 knockout mice and assessed the role of cyclin A2 in liver tumorigenesis. Cyclin A2flox mice (Fig. S1A-C) were crossed to Albumin-Cre transgenic mice to specifically delete cyclin A2 in hepatocytes. Mice with hepatocyte-specific ablation of cyclin A2 (cyclin A2Liv−/−) were viable and did not display any detectable phenotype. Histological analysis of cyclin A2Liv−/− livers revealed a small increase in nuclear size (Fig. S1E), but cyclin A2Liv−/− were otherwise indistinguishable from livers of wild type littermates. It should be noted that the expression of Albumin-Cre begins during embryonic development but the high Cre levels required for full recombination are attained only about 3 weeks after birth, by which time liver development has been completed (21). Tumorigenesis was induced by hydrodynamic tail vein injections of activated Ras in combination with shRNA against p53 (22, 23). Liver tumors in wild type mice could be detected as early as 2 months and all mice examined developed tumors within 4 months. For cyclin A2Liv−/− mice, only one out of 6 mice examined had developed liver tumors by 4 months, while the majority displayed liver tumors after 6 months (Fig. 1A, 1C). Phospho-Erk expression, indicative of Ras activity, could be detected in wild type and knockout livers (Fig. S1F), and was increased in livers with detectable tumors (2 months for wild type and 6 months for cyclin A2Liv−/− livers). Cyclin A2 protein expression could be detected in wild type liver tumors after 2 months but no cyclin A2 was detected in cyclin A2Liv−/− liver tumors as expected (Fig. S1F). Cyclin A2Liv−/− liver tumors expressed Cdk1 in a delayed fashion (6 months onwards, compared to 3 months for wild type liver tumors) but no significant differences were detected in Cdk2 expression between wild type and knockout liver tumors (Fig. S1F). Histological analysis of mouse livers at different time points following induction of tumorigenesis revealed a lower Ki67 index for cyclin A2Liv−/− livers (Fig. 1B-C), indicating impaired tumor cell proliferation in the absence of cyclin A2.
Fig. 1. Delayed liver tumorigenesis in the absence of cyclin A2.
Liver tumors were induced by hydrodynamic tail vein injection of Ras/shRNA p53 in wild type (cyclin A2WT) or liver-specific cyclin A2 knockout (cyclin A2Liv−/−) mice. Tumor formation (assessed over 8 months) is delayed in cyclin A2Liv−/− mice. 4-6 mice were assessed per time point. Wild type mice with tumors were euthanized within 4 months (A, C). “X” indicates that mice with tumors do not survive for 6 months (C). Cyclin A2Liv−/− livers display a decreased Ki67 proliferative index (BC). *p < 0.05, Student’s t-test.
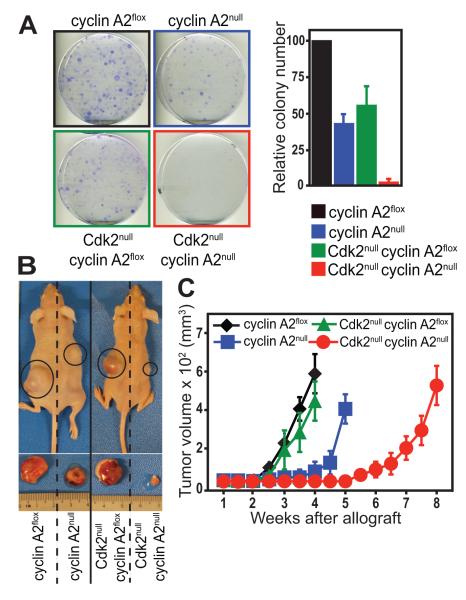
In order to analyze the contribution of Cdk2 in tumors lacking cyclin A2, we chose a transformed mouse embryonic fibroblast (MEF) model lacking both Cdk2 and cyclin A2. We crossed Cdk2 knockout mice (24) and cyclin A2 conditional knockout mice (Fig. S1A-B) to Rosa26-CreERT2 mice, and then intercrossed the resulting knockout strains to obtain MEFs from Cdk2−/−cyclin A2flox/floxRosa26-CreERT2 embryos wherein cyclin A2 loss could be induced by the addition of 4-hydroxytamoxifen [4-OHT] (Fig. S1C-D). Primary Cdk2+/+cyclin A2flox/floxRosa26-CreERT2 or Cdk2−/−cyclin A2flox/floxRosa26-CreERT2 MEFs were transformed using activated Ras and dominant-negative p53 and cyclin A2 loss was induced with 4-OHT. Phospho-Erk expression was assessed to confirm activated Ras signaling (Fig. S2A) and MEFs were treated with Adriamycin to confirm p53 inactivation (Fig. S2B). We analyzed the tumorigenic potential of knockout MEFs by colony formation assays and by subcutaneous transplantation into nude mice. Loss of cyclin A2 or Cdk2 resulted in fewer colonies, while double knockout (DKO) MEFs formed hardly any colonies; only 1-2 colonies per DKO plate were observed (Fig. 2A). Similar results were obtained when MEFs were transformed with activated Ras and c-Myc (Fig. S2C-D). Nude mice transplanted with cyclin A2flox or Cdk2null MEFs transformed with activated Ras and dominant-negative p53 developed tumors of maximal permissible size (20 mm diameter) within 4 weeks (Fig. 2B-C). Cyclin A2null MEFs formed tumors with a 1-2 week delay when compared to cyclin A2flox or Cdk2null MEFs, whereas transformed DKO MEFs exhibited a 4-week delay in tumor formation (Fig. 2B-C). The absence of cyclin A2 in cyclin A2null and DKO tumors was confirmed by Western blot and immunohistochemistry (Fig. S2E-F). Thus, while the loss of Cdk2 did not affect the tumorigenic potential of transformed MEFs, loss of cyclin A2 resulted in a delay in tumor formation. However, combined loss of Cdk2 and cyclin A2 had a more severe effect, resulting in significantly impaired tumorigenesis. We also assessed if deletion of cyclin A2 in growing tumors would have an impact on tumorigenesis (Fig. S3A-B). Nude mice were subcutaneously transplanted with transformed cyclin A2WTRosa26-CreERT2 or cyclin A2floxRosa26-CreERT2 MEFs and tumors were allowed to reach a size of 60-100-mm3 (about 7-10 days after MEF allografts); following which tamoxifen pellets were subcutaneously implanted at a site distant from the tumor. While tamoxifen did not affect the growth of cyclin A2WT tumors (Fig. S3B), cyclin A2flox tumors grew at a decreased rate in the presence of tamoxifen (Fig. S3A). At 4 weeks following MEF transplantation, cyclin A2flox tumors that grew in the presence of tamoxifen displayed a 50% decrease in tumor volume. Genotyping of tumors confirmed Cre-mediated excision of cyclin A2 in the presence of tamoxifen (Fig. S3C).
Fig. 2. Transformed Cdk2nullcyclin A2null MEFs exhibit decreased tumorigenic potential in nude mice.
Cyclin A2flox and Cdk2nullcyclin A2flox MEFs were oncogenically transformed with Ras/p53DN and treated with 4-OHT to induce cyclin A2 knockout. Transformed MEFs were assessed for colony formation (A) or injected subcutaneously into nude mice (B-C). Cdk2nullcyclin A2null MEFs are resistant to transformation (A) and display decreased tumorigenic potential (B-C). Mice in (B) were photographed 4 weeks after MEF allografts. Data is representative of two independent MEF clones and 6 nude mice were used per genotype.
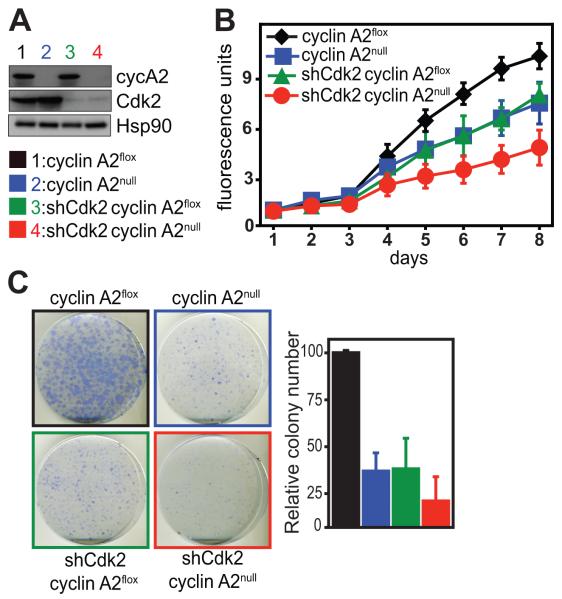
While liver tumorigenesis studies in cyclin A2floxAlbumin-Cre mice revealed a role for cyclin A2 in tumor formation (see Fig. 1), we were interested in testing cyclin A2 inhibition in a more therapeutic context. Hence, we decided to examine whether after tumor formation, inhibition of cyclin A2 (and Cdk2) could impair the proliferation of tumor cells. To this end, we induced liver tumors in cyclin A2floxRosa26-CreERT2 mice by tail vein injections of activated Ras in combination with shRNA against p53. Primary tumor cells were isolated for in vitro culture. The loss of cyclin A2 was induced by addition of 4-OHT, whereas Cdk2 knockdown was achieved using shRNA (Fig. 3A). We assessed the proliferative and colony-forming abilities of these primary liver tumor cells. In comparison to cyclin A2flox cells, cyclin A2null and shCdk2 tumor cells were similarly impaired in proliferation (Fig. 3B) and colony formation (Fig. 3C), while cyclin A2nullshCdk2 tumor cells exhibited the most significant impairment (Fig. 3B-C).
Fig. 3. Impaired proliferation of primary liver tumor cells upon inhibition of cyclin A2 and Cdk2.
Liver tumors were induced by tail vein injection of Ras/shRNA p53 in cyclin A2flox/floxRosa26-CreERT2Tg/Tg mice. Tumors were isolated followed by dissociation and culturing of tumor cells (A-C). Cyclin A2 knockout in primary liver tumor cells was achieved by addition of 4-OHT, whereas Cdk2 was silenced by retroviral shRNA transduction (A). Cyclin A2nullshCdk2 liver tumor cells exhibit decreased proliferation rates as determined by alamarBlue proliferation assay (B) and resistance to colony formation (C). Data in (A-C) is representative of two tumor cell lines established from two mice. NPIU: normalized phosphoimager units.
Concomitant loss of cyclin A2 and Cdk2 in MEFs results in impaired proliferation and premature senescence
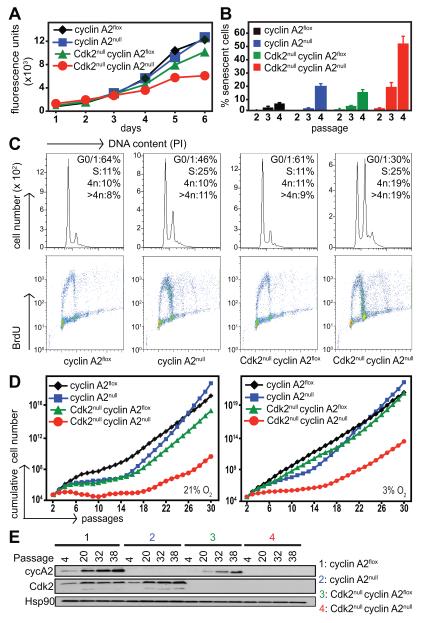
In order to identify the underlying mechanisms of delayed tumorigenesis in the absence of Cdk2 and cyclin A2, we analyzed primary MEFs. In comparison to wild type (cyclin A2flox) MEFs, cyclin A2null MEFs did not display any proliferative defects as described (11), while Cdk2null MEFs displayed a small defect as has been reported previously (24, 25). In contrast, DKO MEFs displayed a significant impairment in proliferation (Fig. 4A). DKO MEFs cultures exhibited flattened morphology characteristic of senescent cells, with the percentage of β-galactosidase positive stained cells increasing after passage 3 (Fig. 4B and S4), indicating that the loss of Cdk2 and cyclin A2 leads to premature senescence. Analysis of the cell cycle distribution in asynchronous MEFs using BrdU labeling and propidium iodide staining revealed an increase in proportion of cells in S phase in cyclin A2null MEFs, while DKO MEFs displayed aberrant distribution of cells with increased population of cells with 4n DNA content (Fig. 4C). In addition to cells in G2/M, the population of cells with 4n DNA content may also represent cells that commit to endo-replication cycles, as seen by the small percentage of polyploid cells [>4n DNA content] (Fig. 4C). Interestingly, the amount of polyploid cells observed in cyclin A2null and DKO MEFs is substantially less compared to what we had observed in Cdk1null MEFs (23). DKO MEFs entered the cell cycle normally from quiescence and also progressed through S phase (Fig. S5) but with a minor delay in entering S phase that has been previously observed in Cdk2null MEFs [(24), Fig. S5] and a minor delay in exiting S phase that was also observed in cyclin A2null MEFs (Fig. S5). This indicates that the presence of Cdk2 and cyclin A2 facilitates efficient progression through S and G2/M phases.
Fig. 4. Concomitant loss of Cdk2 and cyclin A2 results in impaired proliferation and premature senescence.
Primary cyclin A2flox and Cdk2nullcyclin A2flox MEFs were treated with 4-OHT to induce cyclin A2 knockout and their proliferative potential was determined by alamarBlue proliferation assays (A). Cdk2nullcyclin A2null MEFs display impaired proliferation rates when compared to other genotypes. Loss of cyclin A2 and Cdk2 resulted in an increased number of prematurely senescent cells in early passages, as detected by β-galactosidase staining (B). Analysis of cell cycle profile by BrdU labeling and propidium iodide (PI) staining revealed increased population of cells in S phase, and cells with 4n and >4n DNA content in Cdk2nullcyclin A2null cells (C). Data is representative of three independent MEF lines. Cyclin A2flox and Cdk2nullcyclin A2flox MEFs were treated with 4-OHT to induce cyclin A2 knockout and were cultured over several passages using a 3T3 assay at 21% (left panel) and 3% (right panel) oxygen to determine long-term propagative potential (D). Of 7 clones tested, only one Cdk2nullcyclin A2null clone survived the 3T3 assay at 21% oxygen. MEFs were collected at various passages during the course of the 3T3 assay at 21% oxygen conditions and the absence of cyclin A2 protein in cyclin A2null and DKO MEFs was confirmed by Western blotting of protein extracts (E).
MEFs exhibit decreased immortalization rates upon loss of Cdk2 and cyclin A2
Since an essential component of oncogenic transformation is immortalization and Cdk2 in combination with cyclin A2 affected the oncogenicity of transformed MEFs (see Fig. 2), we decided to investigate whether immortalization of MEFs would also be dependent on cyclin A2 and Cdk2. Therefore, we cultured MEFs under either normoxic (21% oxygen) or hypoxic (3% oxygen) conditions using a 3T3 protocol (26) to analyze the long-term proliferative potential of these cells. Both cyclin A2null and Cdk2null MEFs exhibited significantly impaired immortalization rates at early passages that were more prominent under normoxic conditions (Fig. 4D). However, while all cyclin A2null and Cdk2null clones analyzed were able to immortalize eventually, only one out of 7 DKO clones analyzed immortalized under normoxic conditions. After an initial extended lag phase, this DKO clone eventually immortalized after approximately 30 passages (Fig. 4D, left panel). MEFs were analyzed during the course of 3T3 assay to confirm deletion of cyclin A2 in cyclin A2null and DKO MEFs (Fig. 4E). Interestingly, the expression of cyclin A2 was elevated with increasing passage in wild type MEFs.
To fully characterize immortalized DKO MEFs, they were analyzed at passage 30 (P30). The proliferation rates for all genotypes at P30 was comparable to primary MEFs (see Fig. 4A); cyclin A2null MEFs displayed no proliferative defects, Cdk2null MEFs proliferated at a decreased rate, while DKO MEFs displayed the lowest proliferation rate (Fig. S6A). Interestingly, P30 DKO MEFs were unable to survive at low serum conditions (Fig. S6B). While primary DKO MEFs responded appropriately to serum starvation with increased percentage of cells accumulating in G1, P30 DKO MEFs did not accumulate in G1 (Fig. S6C). Re-entry into the cell cycle from quiescence was largely normal for primary DKO MEFs (Fig. S6C, S5), while P30 DKO MEFs exhibited severe apoptosis and extensive polyploidy (Fig. S6C, bottom right panel). While our studies suggest the importance of Cdk2 and cyclin A2 in maintaining cell cycle fidelity in long-term cultures, we also deleted cyclin A2 at P30 in cyclin A2flox or Cdk2nullcyclin A2flox MEFs for a more direct assessment of the role of cyclin A2 in an immortalized system (Fig. S7). Similar to the results obtained with deletion of cyclin A2 at the beginning of the 3T3 assay (see Fig. S6C, right panel), deletion of cyclin A2 in P30 Cdk2nullcyclin A2flox MEFs resulted in apoptosis and increased polyploidy upon re-entry into the cell cycle from quiescence (Fig. S7, bottom panel). It is interesting that while polyploidy in Cdk1null MEFs was driven by high Cdk2/cyclin A2 activity (23), the polyploidy seen here in DKO MEFs is in the absence of Cdk2 and cyclin A2. The underlying mechanism is unclear and we can only speculate that it may be based on activation of Cdc7 or other factors that induce DNA replication.
Cdk1 kinase activity is increased upon loss of cyclin A2
The largely normal cell cycle progression in cyclin A2null MEFs has been attributed to the compensatory role of cyclin E (11) and normalcy in Cdk2null MEFs is due to the compensatory functions of Cdk1 and Cdk4 (27-29). To understand whether these or other cell cycle regulators were affected in primary DKO MEFs, we determined protein levels (Fig. S8A) and kinase activity (Fig. 5A-B, S8B) of various Cdks and cyclins at different time points following release from serum starvation. Cyclin A2 expression was ablated in cyclin A2null and DKO cells, while Cdk2 was undetectable in Cdk2null and DKO cells. No significant differences were detected in protein levels of other regulators such Cdk1, cyclin B1, cyclin E1, p21, and p27 (Fig. S8A). Since cyclin E has been reported to compensate for loss of cyclin A2 in MEFs (11), we analyzed Cdk2/cyclin E complexes in cyclin A2null MEFs and detected increased complex formation (Fig. S8C). In addition, we detected Cdk2/cyclin B1 complexes at low levels in cyclin A2null MEFs (Fig. S8C).
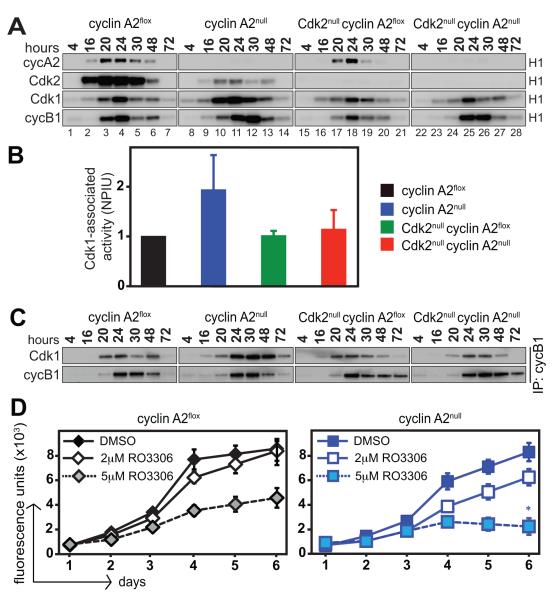
Figure 5. Activity of Cdks in primary MEFs.
Primary MEFs were synchronized at G0/G1 by serum starvation for 72 hours and simultaneously treated with 4-OHT to induce cyclin A2 knockout. MEFs were released into S phase by serum addition and were collected at different time points. Protein extracts were subjected to immunoprecipitation with the indicated antibodies followed by in vitro kinase assays using radiolabeled ATP and histone H1 as substrates. Data is representative of three independent kinase assays performed with three different MEF clones. (A). Quantitative analysis of Cdk1-associated activity is shown as an average of phosphoimager units for three MEF clones at 24, 30, 48, and 72 hours following serum starvation. The average value obtained for cyclin A2flox MEFs was normalized to one and values for the other genotypes were calculated comparatively (B). NPIU: normalized phosphoimager units. Protein extracts were prepared from cells and subjected to co-immunoprecipitation with antibodies against cyclin B1, followed by SDS-PAGE and Western blot with antibodies against Cdk1 (C). Increased Cdk1/cyclin B1 complexes are detected in cyclin A2null MEFs but not in the other genotypes. Synchronized primary MEFs were treated with different concentrations of Cdk1 inhibitor RO3306 and their proliferative potential was determined by alamarBlue proliferation assays. Data is representative of three independent proliferation assays performed with three different MEF clones. (D). Loss of cyclin A2 renders MEFs more sensitive to Cdk1 inhibition. *p < 0.05, Student’s t-test; proliferative rate (slope of graphical trendline) of cyclin A2null MEFs is significantly lower than cyclin A2flox MEFs at 5μM RO3306.
The kinase activity associated with cyclin A2 was not detected in cyclin A2null and DKO MEFs but was slightly decreased in Cdk2null MEFs (Fig. 5A). Cdk2 associated activity was decreased in cyclin A2null (Fig. 5A, lanes 8-14, second panel from top) but not detectable in Cdk2null and DKO MEFs as expected. Importantly, Cdk1 and cyclin B1-associated kinase activities were increased in cyclin A2null MEFs (Fig. 5A: lanes 8-14) but remained unchanged in Cdk2null (Fig. 5A: lanes 15-21) and DKO MEFs (Fig. 5A: lanes 22-28). The increase in Cdk1 activity in cyclin A2null MEFs was statistically significant at 24, 30, 48, and 72 hour time points and peaked at 30 hours following release from serum starvation, where a 2.5-fold increase was observed in comparison to cyclin A2flox MEFs (Fig. S8B). The average of Cdk1-associated kinase activities at 24, 30, 48, and 72 hours following release from serum starvation is presented in Fig. 5B. There was no significant change in the protein expression of Cdk1 and cyclin B1 (see Fig. S8A); however, elevated levels of Cdk1 were bound to cyclin B1 in cyclin A2null MEFs, but such an increase could not be detected in DKO MEFs (Fig. 5C). Since DKO cells exhibit a small degree of polyploidy (see Fig. 4C), it is possible that a fraction of the G2/M cells represent cells that commit to endo-replication cycles and thereby affect the kinetics of Cdk1 activity. Nevertheless, since the overall Cdk1 activity in DKO cells was similar to that of wild type (cyclin A2flox) MEFs (Fig. 5B), we assume that the small proportion of polyploid cells does not significantly confound our interpretation. Finally, a modest increase in Cdk1 activity was also observed upon loss of cyclin A2 in primary liver tumor cells (Fig. S8E), suggesting a previously unappreciated role for Cdk1 activity in the absence of cyclin A2.
To determine the contribution of increased Cdk1 activity to the compensatory mechanisms that prevail in primary cyclin A2null MEFs, we used the Cdk1 inhibitor RO3306 at concentrations that could only partially inhibit Cdk1 activity and still allow for proliferation of MEFs (Fig. 5D). In comparison to control cyclin A2flox cells that proliferated at higher concentrations of RO3306 (5μM), loss of cyclin A2 rendered MEFs more sensitive to Cdk1 inhibition and resulted in decreased (at 2μM RO3306) or ablated proliferation (at 5μM RO3306).
Previous analysis of cyclin A2 in a conditional knockout mouse model revealed it is dispensable in MEFs, but essential for proliferation of hematopoietic and embryonic stem cells. This was attributed to the role of E-type cyclins which are expressed at sufficient levels in MEFs to compensate for absence of cyclin A2, but not in the two stem cell types studied (11). Our findings suggest that in addition to an increase in Cdk2/cyclin E complexes indicative of a compensatory role for cyclin E, there is also an increase in Cdk1 activity upon loss of cyclin A2 in MEFs. Loss of cyclin A2 rendered cells more sensitive to inhibition of Cdk1 activity using low concentrations of Cdk1-specific inhibitor RO3306. Although Cdk1 activity is unchanged in Cdk2null MEFs that exhibit largely normal cell cycle dynamics [Fig. 5A, Fig. S5 and (24)], premature translocation of Cdk1 to the nucleus has been shown to impart near-normalcy in the absence of Cdk2 (29). We provide evidence in this study that in the absence of cyclin A2, Cdk2 activity is significantly decreased (Fig. 5A, l a n e s 8 - 1 4 ) , and Cdk1 activity is increased (Fig. 5A-B). Since loss of either cyclin A2 or Cdk2 alone does not cause any overt defects, this suggests that the compensatory actions of Cdk1 that prevail in cyclin A2null and Cdk2null MEFs are impaired in DKO MEFs.
In summary, we have demonstrated functions for Cdk2 and cyclin A2 in maintaining cell cycle fidelity and regulating tumorigenesis, which appears to be closely related to the regulation of Cdk1 activity. In addition to the loss of Cdk2/cyclin A2 complexes, deletion of cyclin A2 and Cdk2 would result in the loss of Cdk2/cyclin E and Cdk1/cyclin A2 complexes, thus affecting overall Cdk1 and Cdk2 functions. This could account for the exacerbated phenotype observed in DKO cells. We noted that administration of tamoxifen to adult cyclin A2floxRosa-CreERT2 mice resulted in death in around 10 days (average 10.3 ± 4.8 days; n=9) that is associated with very low RBC counts. The essential functions of cyclin A2 in hematopoiesis (11) as well as our results here demonstrating proliferative defects in non-tumor cells upon loss of Cdk2 and cyclin A2 complexes warrant careful design of therapy targeting cyclin A2 functions. Our results may suggest potential benefits of concurrently inhibiting Cdk1 and Cdk2 in cancer therapy.
Supplementary Material
Acknowledgements
We thank Zakiah Talib, June Wang, Vithya Anantaraja, and Chloe Sim for animal care; Shuhui Lim and Matias Caldez for help with nude mice experiments; Elisabeth Pfeiffenberger for help with 3T3 assays and nude mice experiments; Piotr Sicinski for reagents; and the Kaldis lab for support and discussions. We acknowledge the technical expertise provided by the Advanced Molecular Pathology Laboratory at IMCB. We are grateful to Eileen Southon and Susan Reid for help in generating the cyclin A2flox mice. We thank Jos Jonkers and Anton Berns for providing the Rosa26-CreERT2 mice, Mark Lewandoski for the β-actin–Cre/Flpe mice, and T. Jake Liang for the Albumin-Cre mice. This work was supported by the Biomedical Research Council of A*STAR (Agency for Science, Technology and Research), Singapore.
Financial support: This work was primarily funded by the Biomedical Research Council, Singapore; and partly by the Intramural Research Program of the National Institutes of Health, USA.
Footnotes
Conflict of interest: No conflicts to disclose
References
- 1.Girard F, Strausfeld U, Fernandez A, Lamb NJC. Cyclin A is required for the onset of DNA replication in mammalian fibroblasts. Cell. 1991;67:1169–1179. doi: 10.1016/0092-8674(91)90293-8. [DOI] [PubMed] [Google Scholar]
- 2.Pagano M, Pepperkok R, Verde F, Ansorge W, Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992;11:961–971. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yam CH, Fung TK, Poon RYC. Cyclin A in cell cycle control and cancer. Cell Mol Life Sci. 2002;59:1317–1326. doi: 10.1007/s00018-002-8510-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zindy F, Lamas E, Chenivesse X, Sobczak J, Wang J, Fesquet D, et al. Cyclin A is required in S phase in normal epithelial cells. Biochem Biophys Res Commun. 1992;182:1144–1154. doi: 10.1016/0006-291x(92)91851-g. [DOI] [PubMed] [Google Scholar]
- 5.Erlandsson F, Linnman C, Ekholm S, Bengtsson E, Zetterberg A. A detailed analysis of cyclin A accumulation at the G1/S border in normal and transformed cells. Exp Cell Res. 2000;259:86–95. doi: 10.1006/excr.2000.4889. [DOI] [PubMed] [Google Scholar]
- 6.den Elzen N, Pines J. Cyclin A is destroyed in prometaphase and can delay chromosome alignment and anaphase. J Cell Biol. 2001;153:121–136. doi: 10.1083/jcb.153.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geley S, Kramer E, Gieffers C, Gannon J, Peters JM, Hunt T. Anaphase-promoting complex/cyclosome-dependent proteolysis of human cyclin A starts at the beginning of mitosis and is not subject to the spindle assembly checkpoint. J Cell Biol. 2001;153:137–148. doi: 10.1083/jcb.153.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furuno N, den Elzen N, Pines J. Human cyclin A is required for mitosis until mid prophase. J Cell Biol. 1999;147:295–306. doi: 10.1083/jcb.147.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swenson KI, Farrell KM, Ruderman JV. The clam embryo protein cyclin A induces entry into M phase and the resumption of meiosis in Xenopus oocytes. Cell. 1986;47:861–870. doi: 10.1016/0092-8674(86)90801-9. [DOI] [PubMed] [Google Scholar]
- 10.Murphy M, Stinnakre M-G, Senamaud-Beaufort C, Winston NJ, Sweeney C, Kubelka M, et al. Delayed early embryonic lethality following disruption of the murine cyclin A2 gene. Nat Genet. 1997;15:83–86. doi: 10.1038/ng0197-83. [DOI] [PubMed] [Google Scholar]
- 11.Kalaszczynska I, Geng Y, Iino T, Mizuno S, Choi Y, Kondratiuk I, et al. Cyclin A is redundant in fibroblasts but essential in hematopoietic and embryonic stem cells. Cell. 2009;138:352–365. doi: 10.1016/j.cell.2009.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bukholm IR, Bukholm G, Nesland JM. Over-expression of cyclin A is highly associated with early relapse and reduced survival in patients with primary breast carcinomas. International journal of cancer. 2001;93:283–287. doi: 10.1002/ijc.1311. [DOI] [PubMed] [Google Scholar]
- 13.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 14.Chao Y, Shih YL, Chiu JH, Chau GY, Lui WY, Yang WK, et al. Overexpression of cyclin A but not Skp 2 correlates with the tumor relapse of human hepatocellular carcinoma. Cancer Res. 1998;58:985–990. [PubMed] [Google Scholar]
- 15.Odell A, Askham J, Whibley C, Hollstein M. How to become immortal: let MEFs count the ways. Aging. 2010;2:160–165. doi: 10.18632/aging.100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poikonen P, Sjostrom J, Amini RM, Villman K, Ahlgren J, Blomqvist C. Cyclin A as a marker for prognosis and chemotherapy response in advanced breast cancer. Br J Cancer. 2005;93:515–519. doi: 10.1038/sj.bjc.6602735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volm M, Koomagi R, Mattern J, Stammler G. Cyclin A is associated with an unfavourable outcome in patients with non-small-cell lung carcinomas. Br J Cancer. 1997;75:1774–1778. doi: 10.1038/bjc.1997.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolowiec D, Berger F, Ffrench P, Bryon PA, Ffrench M. CDK1 and cyclin A expression is linked to cell proliferation and associated with prognosis in non-Hodgkin’s lymphomas. Leuk Lymphoma. 1999;35:147–157. doi: 10.3109/10428199909145714. [DOI] [PubMed] [Google Scholar]
- 19.Yasmeen A, Berdel WE, Serve H, Muller-Tidow C. E- and A-type cyclins as markers for cancer diagnosis and prognosis. Expert Rev Mol Diagn. 2003;3:617–633. doi: 10.1586/14737159.3.5.617. [DOI] [PubMed] [Google Scholar]
- 20.Chen W, Lee J, Cho SY, Fine HA. Proteasome-mediated destruction of the cyclin a/cyclin-dependent kinase 2 complex suppresses tumor cell growth in vitro and in vivo. Cancer Res. 2004;64:3949–3957. doi: 10.1158/0008-5472.CAN-03-3906. [DOI] [PubMed] [Google Scholar]
- 21.Postic C, Magnuson MA. DNA excision in liver by an albumin-Cre transgene occurs progressively with age. Genesis. 2000;26:149–150. doi: 10.1002/(sici)1526-968x(200002)26:2<149::aid-gene16>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 22.Carlson CM, Frandsen JL, Kirchhof N, McIvor RS, Largaespada DA. Somatic integration of an oncogene-harboring Sleeping Beauty transposon models liver tumor development in the mouse. Proc Natl Acad Sci USA. 2005;102:17059–17064. doi: 10.1073/pnas.0502974102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diril MK, Ratnacaram CK, Padmakumar VC, Du T, Wasser M, Coppola V, et al. Cyclin-dependent kinase 1 (Cdk1) is essential for cell division and suppression of DNA re-replication but not for liver regeneration. Proc Natl Acad Sci USA. 2012;109:3826–3831. doi: 10.1073/pnas.1115201109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berthet C, Aleem E, Coppola V, Tessarollo L, Kaldis P. Cdk2 knockout mice are viable. Curr Biol. 2003;13:1775–1785. doi: 10.1016/j.cub.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 25.Ortega S, Prieto I, Odajima J, Martin A, Dubus P, Sotillo R, et al. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat Genet. 2003;35:25–31. doi: 10.1038/ng1232. [DOI] [PubMed] [Google Scholar]
- 26.Todaro GJ, Green H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aleem E, Kiyokawa H, Kaldis P. Cdc2-cyclin E complexes regulate the G1/S phase transition. Nat Cell Biol. 2005;7:831–836. doi: 10.1038/ncb1284. [DOI] [PubMed] [Google Scholar]
- 28.Berthet C, Klarmann KD, Hilton MB, Suh HC, Keller JR, Kiyokawa H, et al. Combined loss of Cdk2 and Cdk4 results in embryonic lethality and Rb hypophosphorylation. Dev Cell. 2006;10:563–573. doi: 10.1016/j.devcel.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Satyanarayana A, Hilton MB, Kaldis P. p21 inhibits Cdk1 in the absence of Cdk2 to maintain the G1/S phase DNA damage checkpoint. Mol Biol Cell. 2008;19:65–77. doi: 10.1091/mbc.E07-06-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee E-C, Yu D, Martinez de Velasco J, Tessarollo L, Swing DA, Court DL, et al. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics. 2001;73:56–65. doi: 10.1006/geno.2000.6451. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez CI, Buchholz F, Galloway J, Sequerra R, Kasper J, Ayala R, et al. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet. 2000;25:139–140. doi: 10.1038/75973. [DOI] [PubMed] [Google Scholar]
- 32.Lewandoski M, Meyers EN, Martin GR. Analysis of Fgf8 gene function in vertebrate development. Cold Spring Harb Symp Quant Biol. 1997;62:159–168. [PubMed] [Google Scholar]
- 33.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kozar K, Ciemerych MA, Rebel VI, Shigematsu H, Zagozdzon A, Sicinska E, et al. Mouse development and cell proliferation in the absence of D-cyclins. Cell. 2004;118:477–491. doi: 10.1016/j.cell.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 35.Wangensteen KJ, Wilber A, Keng VW, He Z, Matise I, Wangensteen L, et al. A facile method for somatic, lifelong manipulation of multiple genes in the mouse liver. Hepatology. 2008;47:1714–1724. doi: 10.1002/hep.22195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.