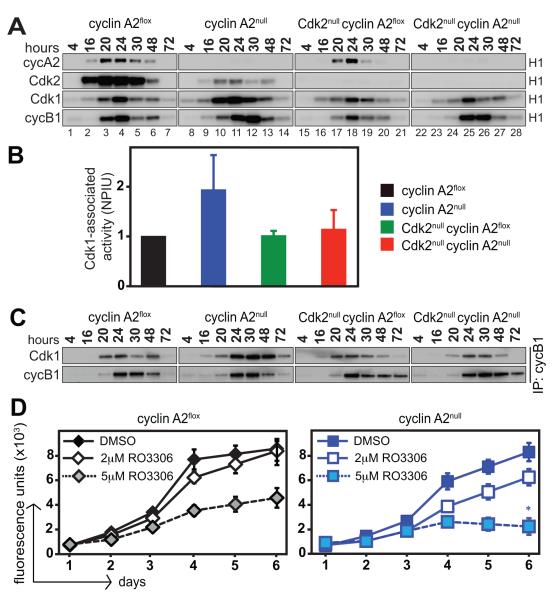
Figure 5. Activity of Cdks in primary MEFs.
Primary MEFs were synchronized at G0/G1 by serum starvation for 72 hours and simultaneously treated with 4-OHT to induce cyclin A2 knockout. MEFs were released into S phase by serum addition and were collected at different time points. Protein extracts were subjected to immunoprecipitation with the indicated antibodies followed by in vitro kinase assays using radiolabeled ATP and histone H1 as substrates. Data is representative of three independent kinase assays performed with three different MEF clones. (A). Quantitative analysis of Cdk1-associated activity is shown as an average of phosphoimager units for three MEF clones at 24, 30, 48, and 72 hours following serum starvation. The average value obtained for cyclin A2flox MEFs was normalized to one and values for the other genotypes were calculated comparatively (B). NPIU: normalized phosphoimager units. Protein extracts were prepared from cells and subjected to co-immunoprecipitation with antibodies against cyclin B1, followed by SDS-PAGE and Western blot with antibodies against Cdk1 (C). Increased Cdk1/cyclin B1 complexes are detected in cyclin A2null MEFs but not in the other genotypes. Synchronized primary MEFs were treated with different concentrations of Cdk1 inhibitor RO3306 and their proliferative potential was determined by alamarBlue proliferation assays. Data is representative of three independent proliferation assays performed with three different MEF clones. (D). Loss of cyclin A2 renders MEFs more sensitive to Cdk1 inhibition. *p < 0.05, Student’s t-test; proliferative rate (slope of graphical trendline) of cyclin A2null MEFs is significantly lower than cyclin A2flox MEFs at 5μM RO3306.