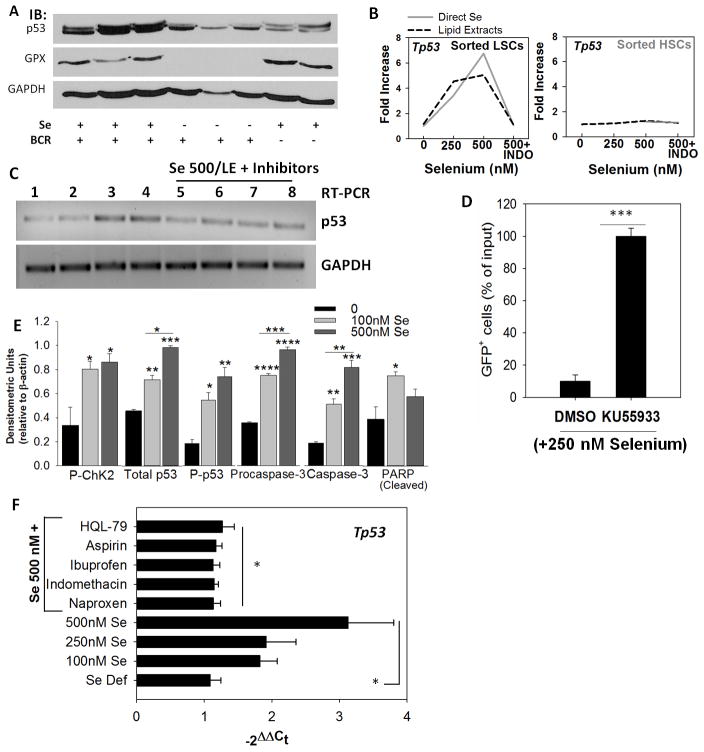
Figure 6.
Activation of the ATM-p53 axis is critical for apoptosis in LSCs. A, western immunoblot showing p53 expression in splenocytes from BCR-ABL+LSC and MSCV-HSC transplanted mice. GPX1 expression was used to confirm selenium status. B, expression of p53 in sorted LSCs and HSCs upon treatment with exogenous selenium (as selenite; solid gray line) or lipid extracts (dotted line) in the presence or absence of indomethacin (INDO). Representative of n= 3–4 independent experiments. C, effect of other NSAIDs on selenium-induced p53 expression as seen by semi-quantitative PCR. Lanes 1–8 represent untreated, 250 nM selenite, 500 nM selenite (as selenite), LE from macrophages (500 nM selenite), LE from 500 nM selenite treated macrophages with INDO, HQL-79, naproxen, and ibuprofen, respectively. D, ATM inhibitor (KU55933; 10 nM) blocks the pro-apoptotic effect of selenite (250 nM) in BCR-ABL+LSCs. Live GFP+ cells following treatment with DMSO (vehicle) or KU55933 are shown in selenite-treated LSCs for 24 h. Mean ± s.e.m. of n= 3 independent experiment; ***p<0.005. E, densitometric analysis of immunoblotting data demonstrating the activation of Chk2-p53 axis in BCR-ABL+LSCs treated with selenite (0–500 nM) for 6 h. All data shown are mean ± s.e.m. of n=5. *, **, ***, **** represent p< 0.05, 0.01, 0.005, 0.001, respectively. F, activation of p53 in peripheral blood sample of AML patient upon treatment with selenium in the presence or absence of NSAIDs. All NSAID treatments were performed on LSCs in the presence of selenite (500 nM selenium). Mean ± s.e.m. * p<0.05.