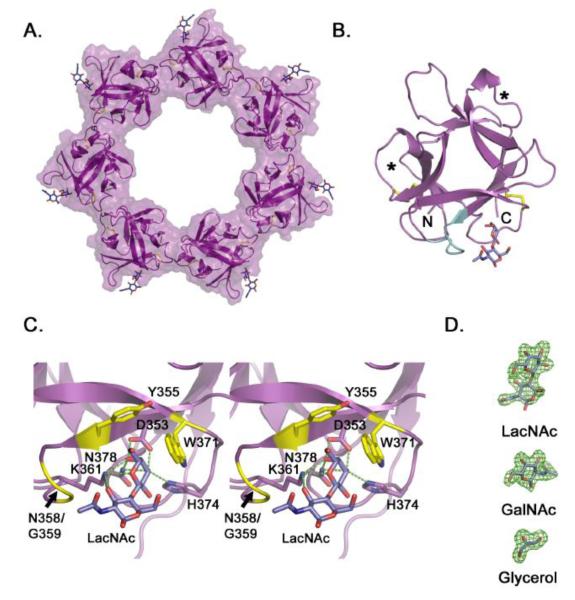
Figure 3. Crystal structure of the VVH β-trefoil lectin.
A. Cartoon representation of the isolated VVH lectin domain crystallized in the presence of LacNAc. Density for LacNAc was clearly interpretable for six of seven subunits, with the remaining LacNAc molecule modeled in to produce this figure. B. Crystal structure of one isolated β-trefoil domain from the heptamer. The two internal disulfide bonds (yellow) are shown in stick representation and the extended loop (Y355-T362) that contacts the ligand is colored in cyan. Asterisks identify the approximate location of carbohydrate binding pockets in β-trefoil domains with multiple binding sites. C. Close-up view of the binding pocket displayed in stereo. Predicted hydrogen bonds are shown as green dotted lines and non-bonded interactions are colored yellow. Predictions were made using the LigPlot+ software 51. D. Electron omit density (green) contoured at 3 σ for representative ligands from the three structures.