Table 2.
EGFR inhibition in the treatment of cervical cancer
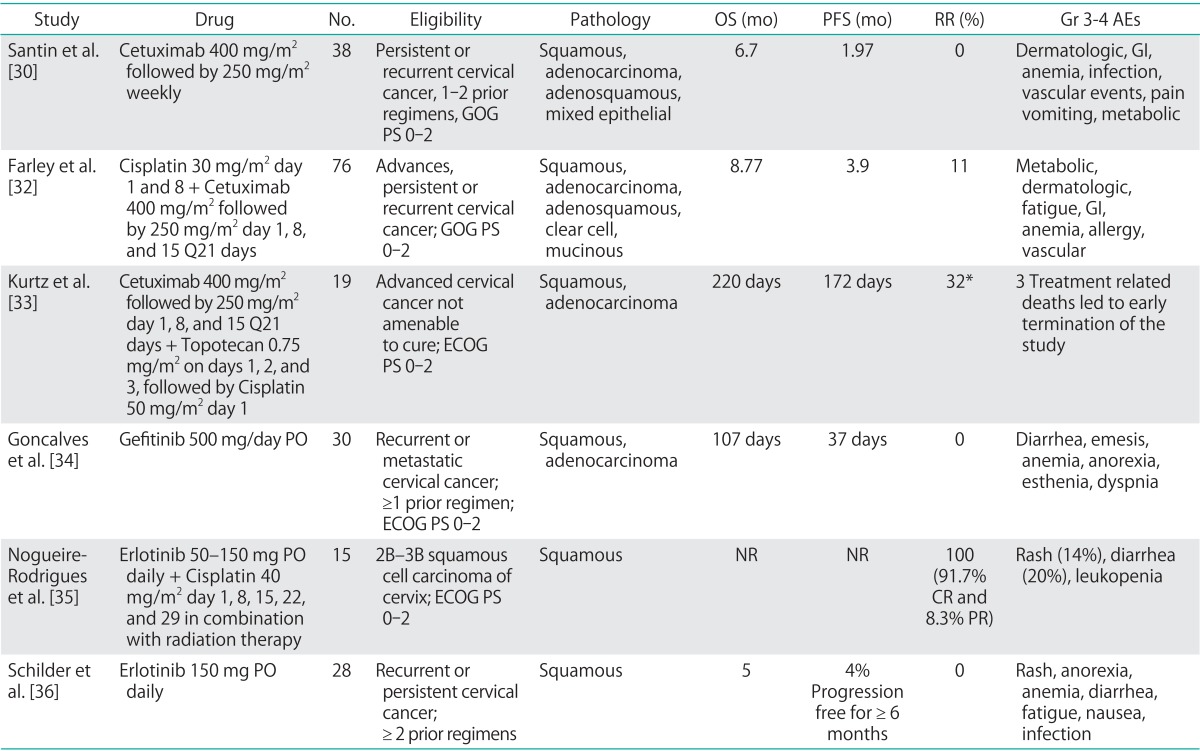
AE, adverse events; CR, complete response; ECOG, European College of Obstetrics and Gynecology; EGFR, epidermal growth factor receptor; GI, gastrointestinal; GOG, Gynecologic Oncology Group; Gr, grade; NR, not reported; OS, overall survival; PFS, progression free survival; PO, per oral; PR, partial response; PS, performance status; RR, response rate.
*Intent to treat analysis in the 19 subjects enrolled on study.
