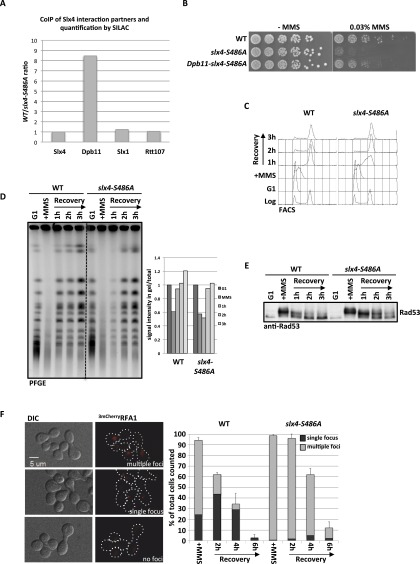
Figure 3.
Mutation of slx4-S486A results in a specific defect in binding to Dpb11 and the response to stalled replication forks. (A) The slx4-S486A mutant leads to a specific defect in binding to Dpb11. Relative enrichment of Slx4 interactors (see Supplemental Fig. S4A) found in purifications of wild-type (WT) Slx43Flag versus Slx4-S486A3Flag as determined by SILAC-based quantitative MS. Values >1 indicate a reduced binding to the Slx4-S486A relative to wild-type Slx4. (B) The slx4-S486A mutant, but not a Dpb11–slx4-S486A-fusion, is hypersensitive to MMS. Wild type or strains expressing slx4-S486A or the Dpb11–slx4-S486A-fusion from the SLX4 promoter as only a copy of SLX4 were spotted in fivefold serial dilutions on MMS-containing medium and assayed for growth after 2 d. (C,D) Replication fork stalling is prolonged in the slx4-S486A mutant. Cells were treated with a pulse of MMS during S phase, and recovery was analyzed by FACS (C; to measure cellular DNA content) and pulsed-field gel electrophoresis (D; to measure intact, fully replicated chromosomes). (D) For quantification, the fluorescence signal of chromosomes that migrated into the gel was divided by the total signal, including the pocket, and all signals were normalized to the G1 sample from each strain. (E) The DNA damage checkpoint is inactivated with reduced kinetics in the slx4-S486A mutant. Cells were treated as in C, and checkpoint activity was determined by anti-Rad53 Western blot. (F) The slx4-S486A mutant shows increased DNA damage foci and delayed recovery after transient MMS treatment in S phase. DNA damage sites were visualized by the ssDNA-binding RFA13mCherry after transient MMS treatment during S phase. Cells were sorted into three categories: multiple, dispersed RFA1 foci; one RFA1 focus; and no RFA1 foci. Values are from two independent experiments, counting 100–150 cells per strain and time point. Error bars represent standard deviations.