Fig. 1.
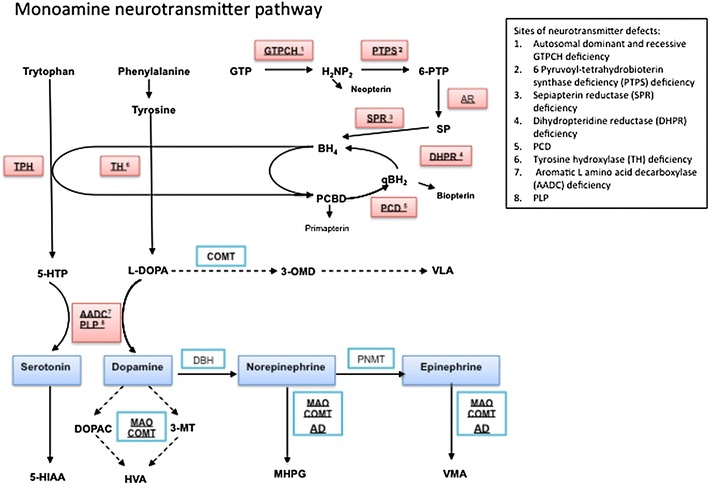
Flow diagram of the monoamine neurotransmitter biosynthesis pathway. The initial substrates for dopamine and serotonin synthesis are aromatic amino acids tyrosine and tryptophan that enter the brain via the large neutral amino acid transporter. They are hydroxylated by tyrosine hydroxylase (TH) and tryptophan hydroxylase (TPH) to levodopa (l-dopa) and 5-hydroxytryptophan (5-HTP), respectively, and are both subsequently decarboxylated by aromatic l-amino acid decarboxylase (AADC) to yield the active neurotransmitters dopamine and serotonin. AADC activity is dependent on its cofactor pyridoxal 5 phosphate. Dopamine and serotonin are further catabolized by monoamine oxidase (MAO) and catechol-O-methyl transferase (COMT) to form 3-methyl 4-hydroxyphenylglycol (MHPG) and homovanillic acid (HVA) from dopamine and 5-hydroxyindoleacetic acid (5-HIAA) from serotonin. These are the stable metabolites measured in the cerebrospinal fluid for neurotransmitter analysis, and are often the key to diagnosis of a childhood monoamine neurotransmitter disorder. l-dopa is metabolised by COMT to 3-OMethyldopa (3-OMD) and then Vanillactic acid (VLA). Both tyrosine hydroxylase and tryptophan hydroxylase activity require the co-factor tetrahydrobiopterin (BH4). Therefore enzymatic deficiencies in the pterin synthesis pathway that affect the levels of this essential cofactor will lead to reduced levels of the monoamine neurotransmitters. BH4 is synthesized in four steps from guanosine triphosphate (GTP) that are dependent on the enzyme activity of guanine triphosphate cyclohydrolase 1 (GTPCH 1), 6-pyruvoyl-tetrahydrobiopterin synthase (PTPS), aldose reductase (AR), and sepiapterin reductase (SPR). The major site of regulation of BH4 biosynthesis is at the level of GTP cyclohydrolase. After coupling as an active co-factor to the aromatic amino hydroxylases (tyrosine and tryptophan hydroxylase), BH4 is regenerated through oxidation by tetrahydrobiopterin-4α-carbinolamine to form quinoid dihydrobiopterin (qBH2) and is subsequently converted back to the active co-factor by dihydrobiopteridine reductase (DHPR). The biogenic amines are illustrated in blue boxes, with the sites of enzyme or cofactor deficiency leading to neurotransmitter disorder highlighted in red boxes with corresponding key for abbreviations used. AD aldehyde dehydrogenase, DOPAC 3,4-dihydroxyphenylacetic acid, DBH dopamine β hydroxylase, GTPCH guanosine triphosphate cyclohydrolase, H 2 NP 2 dihydroneopterin triphosphate, 3-MT 3-methoxytyramine, PCD pterin-4α-carbinolamine dehydratase, PLP pyridoxal phosphate, PNMT phenylethanolamine N-methyltransferase, TH tyrosine hydroxylase, VMA vanillylmandelic acid
