Abstract
Class A scavenger receptor (SR-A) plays an important role in macrophage adhesion. However, the underlying mechanism remains unclear. We previously found that 78 kDa glucose-regulated protein (GRP78) inhibited SR-A-mediated ligand internalization into macrophage by binding to SR-A. The aim of the study was to investigate whether GRP78 could regulate SR-A-mediated cell adhesion. We demonstrated that GRP78 bound directly to SR-A by fluorescence resonance energy transfer (FRET) assay. Overexpression of GRP78 inhibited macrophage adhesion via SR-A. These results suggest that GRP78 may act as an inhibitor of macrophage adhesion via SR-A.
Keywords: class A scavenger receptor, glucose-regulated protein 78 (GRP78), macrophage adhesion, fluorescence resonance energy transfer, 6-aminonicotinamide
INTRODUCTION
Class A scavenger receptor (SR-A) is a trimeric, type II trans-membrane glycoprotein that is initially characterized by internalizing modified low density lipoprotein (LDL). Meanwhile, SR-A participates in the deposition of cholesterol in macrophages in the arterial wall during the development of atherosclerotic lesions[1]. SR-A may, in fact, contribute to atherogenesis in ways distinct from the uptake of modified lipoproteins. For instance, SR-A plays an important role in immune response and cell adhesion. SR-A may interact with components of the subendothelial space, thereby contributing to the adhesion and retention of macrophages in the artery wall[2]. Using SR-A-specific monoclonal antibody and SR-A knockout mice, it has been demonstrated that SR-A is essential for divalent cation-independent macrophage adhesion[3],[4]. That SR-A mediated adhesion may play an important role in vivo is suggested by the demonstration of increased macrophage accumulation and enhanced granuloma formation in transgenic mice overexpressing SR-A[5]. Several components of the extracellular matrix, including modified types of collagen and certain proteoglycan present at sites of inflammation, have been identified as adhesion substrates for SR-A[2]. Thus, SR-A-mediated macrophage adhesion and the ensuing macrophage retention and activation may play important roles in chronic inflammation associated with atherosclerosis.
Cell adhesion is a complex process that involves initial attachment of cells to substrate and subsequent induction of a spread morphology that is characterized by an increase in surface area and organization of the actin cytoskeleton. SR-A induces similar changes and mediates cell adhesion via the activation of several intracellular signaling molecules. Activation of these signaling pathways results in the formation of focal adhesions and cytoskeletal changes that promote cell adhesion[6]-[8]. However, the modulation of SR-A-mediated adhesion has not been clearly defined. Recently, we identified 78 kDa glucose-regulated protein (GRP78) as a novel binding partner for SR-A and GRP78 inhibited SR-A-mediated internalization of acetylated LDL[9]. Thus, we hypothesized that GRP78 may also regulate SR-A-dependent macrophage adhesion.
In the present study, we confirmed that GRP78 could bind directly to SR-A as evidenced by fluorescence resonance energy transfer (FRET) assay, and inhibit macrophage adhesion via SR-A. Therefore, our data lends further support to the role of GRP78 as a novel modulator of SR-A-dependent pathophysiological processes in macrophages.
MATERIALS AND METHODS
Cell culture and transfection
THP-1 cells (American Type Culture Collection, ATCC, Manassas, VA, USA) were cultured in Roswell Park Memorial Institute medium 1640 (RPMI-1640, Hyclone, Logan, UT, USA) containing 10% (v/v) fetal calf serum (FCS, Hyclone, USA), and supplemented with 2 mmol/L L-glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin. Phorbol 12-myristate 13-acetate (PMA, Sigma, St Louis, MO, USA, 100 nmol/L) was added to THP-1 cells for 3 days to induce a macrophage phenotype of differentiation. Human embryonic kidney cells (HEK 293, ATCC, USA) were maintained in Dulbecco's modified Eagle's medium (DMEM, Hyclone, Logan, UT, USA) and transfected with plasmids and Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instruction.
Plasmid construction
The cDNA coding for human SR-A was obtained from EGFP-SR-A (a gift from Dr. Harald Heider, University of Basel, Switzerland) by PCR (5′-CCCAAGCTTGGATGGAGCAGTGGGATC-3′; 5′-CGCGGATCCTTAATGTGTTTCCACTCC-3′). The cDNA coding for human GRP78 was obtained from pcDNA3.1-GRP78 (a gift from Dr. Peter Bross, University of Aarhus, Denmark) by PCR (5′-CCCAAGCTTGCAAGATGAAGCTCTCCC-3′; 5′-CGCGGATCCAACTCATCTTTTTCTGC-3′). The amplified cDNA was digested with the appropriate restriction enzymes and then cloned into a similarly digested vector.
Western blotting assays
Cell lysates or immunoprecipitates were separated by 10% SDS-PAGE. Proteins were transferred to a PVDF membrane and blocked for 30 minutes in blocking buffer (tris-buffered saline, pH 7.6, 0.05% Tween and 3% BSA). After incubation with primary antibody diluted in blocking buffer for 60 minutes and washing, the blot was incubated for 30 minutes with appropriate secondary anti-IgG horseradish peroxidase conjugate. The membrane was washed three times for 10 minutes each and developed with Super Signal chemiluminescent substrate (Pierce, Rockford, IL, USA). The primary antibodies against SR-A (Santa Cruz Biotechnology, Santa Cruz, CA, USA), GRP78 (Sigma, St. Louis, MO, USA) and β-actin (Santa Cruz Biotechnology) were used.
Fluorescence resonance energy transfer assay
Cells co-transfected with yellow fluorescent protein (YFP)-GRP78 and cyan fluorescent protein (CFP)-SR-A were imaged on an Olympus IX81 inverted epifluorescent microscope equipped with an Evolution QEi camera, imaging software (Image-Pro AMS 5.1) and a 100×oil objective. Three-cube fluorescence resonance energy transfer (FRET) filter-cubes (excitation, dichroic, emission) were CFP (D440/20M, 455DCLP, D480/30M, Chroma), YFP (500RDF25, 525DRLP, 530EFLP, Omega Optical), and fluorescence resonance energy transfer (FRET, 440DF20, 455DRLP, 535DF25, Omega Optical). When cells were observed under the FRET filter set, leak of the CFP emission through the FRET filter and excitation of YFP directly by the FRET excitation wavelength took place. To eliminate these interferences, the following calculation was used, FRETC = I FRET-CCFP-CYFP (Eq. 1) where I FRET is the intensity of FRET with the FRET filter set of CFP/YFP-co-expressed cells, CCFP and CYFP are the contributions from CFP and YFP, respectively. In our experiments, CCFP = 0.2 × ICFP and CYFP = 0.19 × IYFP (Eq. 2) where ICFP is the intensity of CFP observed under the CFP filter set and IYFP is the intensity of YFP observed under the YFP filter set in co-expressing cells. The FRET was then calculated by FRETC = IFRET - 0.2 × ICFP - 0.19 × IYFP. Cells co-transfected with YFP and CFP vectors were imaged as negative controls and cells transfected with YFP-CFP fusion plasmids were imaged as positive controls. The mean FRET ratio (FR) was used as a parameter for comparison. FR = (IFRET - 0.2 × ICFP)/(0.19 × IYFP) (Eq. 3)
Cell adhesion assays
PMA-elicited THP-1 cells were obtained by centrifugation, re-suspended in RPMI 1640 medium, and counted with a cell counter. Macrophages were seeded in wells, incubated for 30 minutes at 37 °C, and washed three times with PBS. Adhered cells were incubated in ethylene diamine tetraacetic acid (EDTA) solution (0.2 mg/mL, 37 °C) for 10 minutes to eliminate divalent cation-dependent adhesion. Following incubation, non-attached cells were removed by washing, adherent cells were fixed with 4% paraformaldehyde and stained with 4',6-diamidino-2-phenylindole (DAPI, Molecular Probes, Eugene, OR, USA). Fluorescence images were captured and then the nuclei in each image were counted.
Statistical analysis
Data were expressed as mean ± SD. Statistical significance between the groups was assessed by Student's t test or one-way analysis of variance (ANOVA) followed by pairwise comparison with a post-hoc test with Bonferroni correction. P < 0.05 was considered statistically significant.
RESULTS
Construction and transfection of CFP-SR-A and YFP-GRP78 vectors
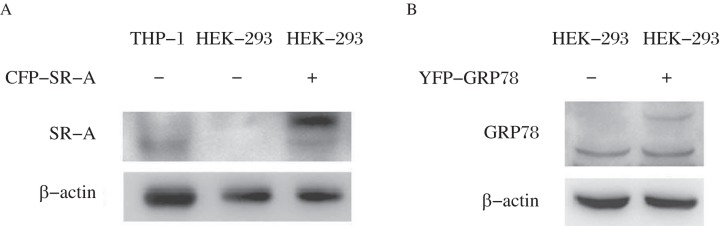
The cDNAs of SR-A and GRP78 were amplified from the EGFP-SR-A and pcDNA3.1-GRP78 plasmids, respectively, and then inserted into CFP- and YFP-tagged vectors. PCR analysis of the cloned plasmids followed by gene sequencing showed the full-length sequences of SR-A and GRP78 were successfully and correctly cloned into the vectors. Furthermore, high levels of CFP-SR-A or YFP-GRP78 were detected by Western blotting assay in HEK293 cells transfected with CFP-SR-A or YFP-GRP78 (Fig. 1).
Fig. 1. Western blotting assays of CFP-SR-A and YFP-GRP78 expression in HEK 293 cells.
HEK 293 cells were transfected with CFP-SR-A (A) or YFP-GRP78 plasmid (B), and further analyzed by Western blotting assay with anti-SR-A or anti-GRP78 antibody. PMA treated THP-1 cells were performed as positive control.
GRP78 binds directly to SR-A
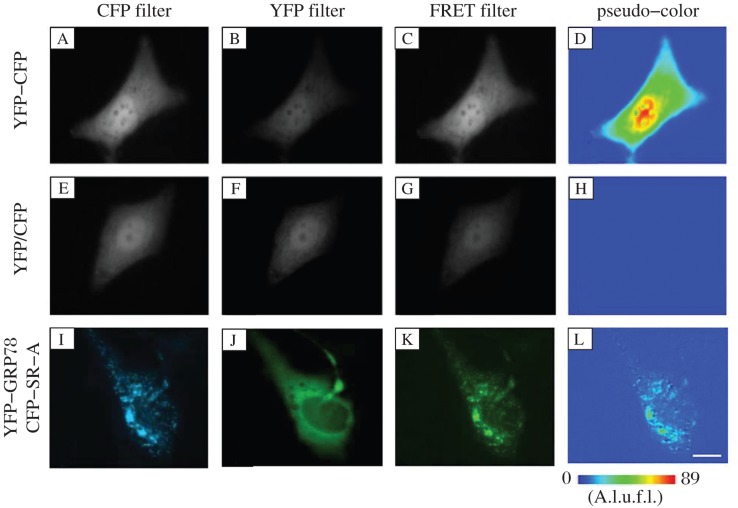
The direct interaction between GRP78 and SR-A in living cells was demonstrated by FRET assay. When HEK293 cells were co-transfected with YFP-GRP78 and CFP-SR-A plasmids, the mean FRET ratio (FR) of YFP-CFP was 4.4165 and YFP/CFP was 1.0085. The mean FR of YFP-GRP78/CFP-SR-A was 2.1700, which was statistically different from that of YFP/CFP (Table 1). Morphological observation from the YFP or CFP channel revealed co-localization of GRP78 and SR-A in the cytoplasm and on cell membranes (Fig. 2I and 2J). When 33 groups of YFP-GRP78/CFP-SR-A-co-transfected cells were imaged with YFP, CFP, or FRET channel, respectively, the mean ratio of YFP-GRP78 to CFP-SR-A was 0.30 (Fig. 2I, 2J and 2K). Measurements of sensitized FRET efficiencies between CFP and YFP (FRETC) on a pixel-by-pixel basis revealed a strong energy transfer between YFP-GRP78 and CFP-SR-A (Fig. 2L), which strongly indicates a direct interaction between these two proteins in living cells.
Table 1. Values of FRETR, FR, and YFP/CFP in HEK 293 cells transfected with YFP/CFP plasmids, YFP-CFP fusion plasmid, and CFP-SR-A/YFP-GRP78 plasmids.
| YFP/CFP (n = 8) | YFP-CFP (n = 8) | YFP-GRP78/CFP-SR-A (n = 33) | |
| FRETR | 1.0023±0.0289 | 2.2546±0.1789 a | 1.2547±0.0658 b |
| FR | 1.0085±0.1947 | 4.4165±0.5157 a | 2.1700±0.3792 b |
| YFP/CFP | 0.1–0.45 | 0.4519±0.0386 | 0.2951±0.0752 |
a, P < 0.05, compared with YFP/CFP; b, P < 0.05, compared with YFP-CFP.
Fig. 2. GRP78 interacts directly with SR-A.
HEK 293 cells transfected with CFP-SR-A and YFP-GRP78 were imaged with CFP (CFP filter lane), YFP (YFP filter lane), and FRET (FRET filter lane) channel at room temperature (I,J,K). FRETC was calculated as described in Methods and represented as a pseudo-color image (pseudo-color lane) (l). Cells transfected with YFP-CFP fusion plasmid were used as positive controls (A,B,C,D). The YFP/CFP plasmids-transfected cells were negative controls (E,F,G,H). A.l.u.f.i., arbitrary linear units of fluorescence intensity. Bar, 10 μm.
Overexpression of GRP78 inhibits SR-A-mediated macrophage adhesion
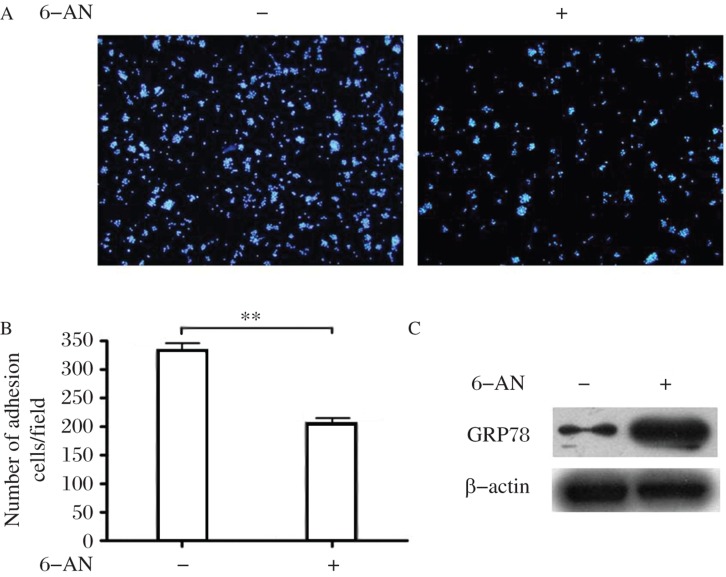
It has been demonstrated that SR-A is important for divalent cation-independent macrophage adhesion. When EDTA was added to chelate divalent cations, SR-A mediated 95% of the macrophage adhesion[3],[4]. We next investigated whether the upregulation of GRP78 could impact SR-A-mediated cell adhesion macrophages. Therefore, we employed 6-aminonicotinamide (6-AN), an analogue of niacin and a weak inhibitor of poly (ADP-ribose) polymerase that has been shown to specifically up-regulate GRP78 in cells[10]. THP-1 macrophages were treated with 6-AN and maintained in the medium for additional 4 hours to allow the nicotinamide-adenine dinucleotide phosphate (NAD(P)) metabolism to return to normal. Western blotting assay showed that GRP78 expression was significantly upregulated in the treated cells (Fig. 3C). Importantly, cell adhesion analysis showed that 6-AN-treated macrophages exhibited a significant 40% reduction in adhesion as compared with that of PBS-treated cells (Fig. 3A and 3B). These data suggested that GRP-78 negatively regulates SR-A dependent cell adhesion.
Fig. 3. Overexpression of GRP78 attenuates SR-A mediated THP-1 cell adhesion.
A: After treatment with 0.2 mmol/L 6-AN for 36 hours, PMA-differentiated THP-1 cells were treated as described in Methods, and adherent cells were fixed and stained with DAPI. B: Comparison of adherent cell number between 6-AN treated THP-1 cells and controls. Five representative fields were photographed in each group. Experiments were performed in triplicate. **P < 0.01 compared with controls. C: Western blotting assays of GRP78 in 6-AN treated THP-1 cells and controls. 6-AN: 6-aminonicotinamide.
DISCUSSION
GRP78 is an endoplasmic reticulum (ER) resident molecular chaperone. In the ER, it could direct protein folding and assembly, contribute to ER calcium homeostasis, and activate the mammalian unfolded protein response. Additionally, it plays a role in protecting cells against apoptosis[11],[12]. GRP78 was previously identified as a novel binding protein of SR-A, and the binding of GRP78 to SR-A inhibited the internalization of acetylated LDL and reduced lipid accumulation in macrophages[9]. In the present study, we demonstrate that GRP78 interacts with SR-A directly and inhibits the SR-A-mediated macrophage adhesion.
Our previous study employed glutathione S-transferase (GST) pull-down, immunoprecipitation and immunocytochemical analyses to confirm the interaction between GRP78 and SR-A. FRET assay is a new technique for probing protein-protein interaction in live cells. FRET relies on the distance-dependent transfer of energy from a donor molecule to an acceptor molecule. The donor and acceptor molecules must be in close proximity to one another (typically 10–100 Å). Due to its sensitivity to distance, FRET has been used to investigate molecular interactions. In the present study, CFP-SR-A and YFP-GRP78 expression vectors were constructed and applied to FRET analysis. Measurements of sensitized FRET efficiencies between CFP and YFP revealed a strong energy transfer between YFP-GRP78 and CFP-SR-A, which strongly indicates a direct interaction between GRP78 and SR-A in live cells.
It has been reported that SR-A mediated macrophage adhesion requires sequential activation of Lyn and PI3-kinase and coupling of calcium-independent phospholipase A2 and 12/15-lipoxygenase to Rac and Cdc42 activation[6],[7]. But it is unclear if GRP78 may inhibit SR-A-mediated cell adhesion. It has been found that IRE1 is a trans-membrane protein kinase in the ER that could transmit stress signals in response to protein misfolding. In normal cells, GRP78 binds to the luminal domains of IRE1. The protein misfolding promotes the dissociation of GRP78 from IRE1 and activates IRE1-mediated signaling pathways[13]. We propose a similar model for SR-A-GRP78 interaction. Normally, GRP78 may bind to SR-A to inhibit the downstream signaling pathway, leading to diminished cell adhesion. When GRP78 becomes dissociated from SR-A, the SR-A-mediated signaling pathway is activated with enhanced cell adhesion.
In atheroprone areas of the vasculature, upregulation of GRP78 was detected before the initiation of atherosclerosis[14]. Overexpression of GRP78 may provide novel methods to inhibit the functions of macrophage SR-A. As an inhibiting protein for SR-A, GRP78 may play a role in suppressing the development of atherosclerosis, as it reduces macrophage adhesion that occurs at all stages of atherosclerosis. In summary, our data have confirmed the presence of an SR-A-GRP78 complex in macrophages that serves to regulate cell adhesion. These findings open a new avenue of research that traces the behavior of macrophages during atherogenesis and will likely provide novel target for the intervention and prevention of atherosclerosis.
References
- 1.Murphy JE, Tedbury PR, Homer-Vanniasinkam S, Walker JH, Ponnambalam S. Biochemistry and cell biology of mammalian scavenger receptors. Atherosclerosis. 2005;182:1–15. doi: 10.1016/j.atherosclerosis.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 2.Kzhyshkowska J, Neyen C, Gordon S. Role of macrophage scavenger receptors in atherosclerosis. Immunobiology. 2012;217:492–502. doi: 10.1016/j.imbio.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Fraser I, Hughes D, Gordon S. Divalent cation-independent macrophage adhesion inhibited by monoclonal antibody to murine scavenger receptor. Nature. 1993;364:343–6. doi: 10.1038/364343a0. [DOI] [PubMed] [Google Scholar]
- 4.Santiago-Garcia J, Kodama T, Pitas RE. The class A scavenger receptor binds to proteoglycans and mediates adhesion of macrophages to the extracellular matrix. J Biol Chem. 2003;278:6942–6. doi: 10.1074/jbc.M208358200. [DOI] [PubMed] [Google Scholar]
- 5.Daugherty A, Kosswig N, Cornicelli JA, Whitman SC, Wolle S, Rateri DL. Macrophage-specific expression of class A scavenger receptors enhances granuloma formation in the absence of increased lipid deposition. J Lipid Res. 2001;42:1049–55. [PubMed] [Google Scholar]
- 6.Nikolic DM, Cholewa J, Gass C, Gong MC, Post SR. Class A scavenger receptor-mediated cell adhesion requires the sequential activation of Lyn and PI3-kinase. Am J Physiol Cell Physiol. 2007;292:C1450–8. doi: 10.1152/ajpcell.00401.2006. [DOI] [PubMed] [Google Scholar]
- 7.Nikolic DM, Gong MC, Turk J, Post SR. Class A scavenger receptor-mediated macrophage adhesion requires coupling of calcium-independent phospholipase A(2) and 12/15-lipoxygenase to Rac and Cdc42 activation. J Biol Chem. 2007;282:33405–11. doi: 10.1074/jbc.M704133200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cholewa J, Nikolic D, Post SR. Regulation of class A scavenger receptor-mediated cell adhesion and surface localization by PI3K: identification of a regulatory cytoplasmic motif. J Leukoc Biol. 2010;87:443–9. doi: 10.1189/jlb.0509318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ben J, Gao S, Zhu X, Zheng Y, Zhuang Y, Bai H, et al. Glucose-regulated protein 78 inhibits scavenger receptor A-mediated internalization of acetylated low density lipoprotein. J Mol Cell Cardiol. 2009;47:646–55. doi: 10.1016/j.yjmcc.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Jorgensen MM, Jensen ON, Holst HU, Hansen JJ, Corydon TJ, Bross P, et al. Grp78 is involved in retention of mutant low density lipoprotein receptor protein in the endoplasmic reticulum. J Biol Chem. 2000;275:33861–8. doi: 10.1074/jbc.M004663200. [DOI] [PubMed] [Google Scholar]
- 11.Hendershot LM. The ER function BiP is a master regulator of ER function. Mt Sinai J Med. 2004;71:289–97. [PubMed] [Google Scholar]
- 12.Ni M, Zhang Y, Lee AS. Beyond the endoplasmic reticulum: atypical GRP78 in cell viability, signalling and therapeutic targeting. Biochem J. 2011;434:181–8. doi: 10.1042/BJ20101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–32. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 14.Zhou J, Lhotak S, Hilditch BA, Austin RC. Activation of the unfolded protein response occurs at all stages of atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation. 2005;111:1814–21. doi: 10.1161/01.CIR.0000160864.31351.C1. [DOI] [PubMed] [Google Scholar]