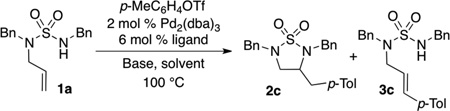
Table 1.
Optimization of Aryl Triflate Carboamination[a]
 | |||||
|---|---|---|---|---|---|
| Entry | Ligand | Base | Solvent | 2c:3c | Conversion (%)[b] |
| 1 | X-Phos | NaOtBu | toluene | 1:1 | 81 |
| 2 | RuPhos | NaOtBu | toluene | 9:1 | 90 |
| 3 | RuPhos | LiOtBu | toluene | 19:1 | 100 |
| 4 | RuPhos | LiOtBu | PhCF3 | >25:1 | 100[c] |
Reaction Conditions: 1.0 equiv 1a, 1.2 equiv p-MeC6H4OTf, 1.4 equiv base, 2 mol % Pd2(dba)3, 6 mol % ligand, solvent (0.25 M), 100 °C.
Conversion = percentage of starting material consumed.
The reaction was conducted using 2 mol % Pd(OAc)2 and 5 mol % ligand.
