Table 2.
Pd-Catalyzed Carboamination of N-Allyl Sulfamides [a]
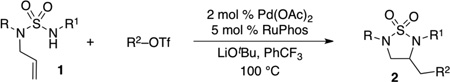 | |||||
|---|---|---|---|---|---|
| Entry | R | R1 | R2 | Product | Yield (%)[b] |
| 1 | Bn | Bn | p-Me-C6H4 | 2c | 85 |
| 2 | Bn | Bn | p-NC-C6H4 | 2a | 90 |
| 3 | Bn | Bn | p-MeO-C6H4 | 2d | 90 |
| 4 | Bn | Bn | o-Me-C6H4 | 2e | 85 |
| 5 | Bn | Bn | 1-cyclohexenyl | 2f | 87[c] |
| 6 | Bn | Bn | E-1-decenyl[e] | 2g | 80[d,e,f] |
| 7 | Me | Bn | p-Cl-C6H4 | 2h | 79 |
| 8 | Bn | PMB | p-Me-C6H4 | 2i | 90 |
| 9 | Bn | Me | m-F3C-C6H4 | 2j | 86 |
| 10 | Bn | tBu | p-MeO-C6H4 | 2k | 92 |
| 11 | Bn | PMP | Ph | 2l | 90[g] |
| 12 | Me | Bn | 2m | 84 | |
| 13 | tBu | Bn | m-F3C-C6H4 | 2n | 88[h] |
| 14 | H | allyl | Ph | 2o | 51[i] |
Reaction Conditions: 1.0 equiv 1, 1.2 equiv R2OTf, 1.4 equiv LiOtBu, 2 mol % Pd(OAc)2, 5 mol % RuPhos, PhCF3 (0.25 M), 100 °C.
Isolated yield (average of two experiments).
The reaction was conducted using Brettphos as ligand.
The reaction was conducted using tBu-Davephos as ligand.
The alkenyl triflate was used as a 5:1 mixture of E:Z isomers, and the product was obtained as a 5:1 E:Z mixture.
The reaction was conducted using 1.4 equiv R2OTf and 1.6 equiv LiOtBu.
The reaction was conducted using tBu-X-Phos as ligand.
The reaction was conducted using 7.5 mol % ligand.
The reaction was conducted using 2.4 equiv R2OTf and 2.4 equiv LiOtBu.
