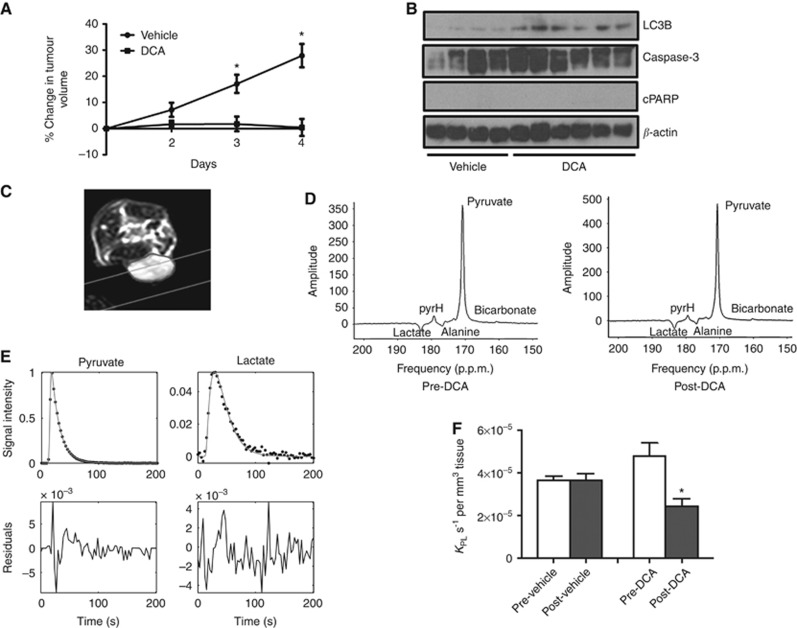
Figure 8.
In vivo response to DCA treatment in HT29 xenografts. (A) Changes in HT29 tumour volumes (relative to day 1) following 4 days of treatment with vehicle (n=6) or 200 mg kg−1 DCA via p.o. (n=7). Data are expressed as mean±s.e.m. *P⩽0.005. (B) Western blots of LC3B, caspase-3, cleaved PARP and β-actin in HT29 xenografts following 4 days of vehicle (saline, n=4) or 200 mg kg−1 DCA treatment (n=6) via p.o. (C) Transverse image of a mouse with a subcutaneous HT29 xenograft (tumour outlined). The grey lines indicate the acquisition slice for 13C-MRS. (D) Slice selective in vivo hyperpolarised 13C-MR spectra acquired using a surface coil on a 3 T clinical scanner from a HT29 xenograft pre- and post-DCA treatment. Displayed spectra are the sum of the first 24 spectra from a dynamic series acquired using a 20° flip angle. There is evidence of a small bicarbonate peak at 161 p.p.m., both pretreatment and posttreatment, but it was not large enough to be included in the kinetic modelling. (E) Representative in vivo pyruvate and lactate signal areas acquired from Amares fitting in jMRUI, and three-site Matlab kinetic model fitting (solid lines) were used to obtain the apparent rate constants of pyruvate-to-lactate exchange. (F) Apparent forward rate constant of pyruvate-to-lactate exchange kPL for saline- (mean±s.e.m., n=3) and DCA-treated (mean±s.e.m., n=3) mice bearing subcutaneous HT29 xenografts. DCA treatment induced a significant 49% reduction in kPL (*P=0.01, paired, two-tailed Student's t-test). There was no significant change in kPL between prescans and postscans in the vehicle group.