Abstract
Background:
The incidence of malignant pleural mesothelioma (MPM) in elderly patients is increasing. There are no specific guidelines for their management.
Methods:
The clinical records of elderly patients (⩾70 years old) with MPM referred from January 2005 to November 2011 to six Italian Centres were reviewed. Age, gender, histology, International Mesothelioma Interest Group (IMIG) stage, Eastern Cooperative Oncology Group Performance Status (ECOG-PS), Charlson Comorbidity Index (CCI) and treatment modalities were analysed and correlated to overall survival (OS).
Results:
In total, 241 patients were identified. Charlson Comorbidity Index was ⩾1 in 92 patients (38%). Treatment was multimodality therapy including surgery in 18, chemotherapy alone in 180 (75%) and best supportive care in 43 cases (18%). Chemotherapy was mainly pemetrexed based. Median OS was 11.4 months. Non-epithelioid histology (HR 2.32; 95% CI 1.66–3.23, P<0.001), age ⩾75 years (HR 1.44; 95% CI 1.08–1.93, P=0.014), advanced (III–IV) stage (HR 1.47; 95% CI 1.09–1.98, P=0.011) and CCI⩾1 (HR 1.38; 95% CI 1.02–1.85, P=0.034) were associated to a shorter OS. Treatment with pemetrexed was associated with improved OS (HR 0.40; 95% CI 0.28–0.56, P<0.001).
Conclusions:
Non-epithelioid histology, age ⩾75 years, advanced IMIG stage and presence of comorbidities according to CCI were significant prognostic factors in elderly patients with MPM. Treatment with pemetrexed-based chemotherapy was feasible in this setting. Prospective dedicated trials in MPM elderly patients selected according to prognostic factors including comorbidity scales are warranted.
Keywords: pleural mesothelioma, comorbidity, Charlson Index, elderly, pemetrexed
The incidence of malignant pleural mesothelioma (MPM) is rising in most parts of the world, as a consequence of widespread exposure to asbestos (Delgermaa et al, 2011; Park et al, 2011; Robinson, 2012; Diandini et al, 2013). Owing to the long latent period following exposure, MPM is often diagnosed late in life. An increasing proportion of elderly patients is reported by several epidemiological studies and mesothelioma registers in industrialised countries (Hodgson et al, 2005; Clements et al, 2007; Price and Ware, 2009; Myojin et al, 2012; Marinaccio et al, 2012; Magnani et al, 2013). However, there are no specific guidelines for their management. As a consequence, clinical uncertainty often results in suboptimal or excessively toxic treatments, which ultimately may lead to poorer outcomes as compared with younger patients (Dale, 2003; de Magalhães, 2013). Although geriatric oncology has definitely been accepted as a defined field of clinical activity and research (Lichtman et al, 2007; Hamaker et al, 2012), elderly patients with relatively rare tumours such as MPM are under-represented in clinical trials. Median age of patients enrolled in the cisplatin/pemetrexed phase III trial, which has established the current standard of systemic therapy for MPM, was 61 years (Vogelzang et al, 2003). In that trial, the percentage of patients aged ⩾70 years was about 19% (Vogelzang and Symanowski, personal communication). Schedules with carboplatin have been implemented for MPM patients who are unfit to receive cisplatin; however, the proportion of elderly patients in these trials remained low, ranging from 21 to 34%, with median age of study populations of 65–67 years (Ceresoli et al, 2006, 2013; Castagneto et al, 2008). Prospective as well as retrospective data regarding prognostic factors and the efficacy and tolerability of anticancer treatments in elderly patients affected by MPM are lacking. The aim of this study was to retrospectively evaluate the diagnostic and therapeutic management of a large series of elderly MPM patients referred to six Italian Centres in a 7-year span after the availability of pemetrexed, focusing on the factors affecting their survival, including comorbidity.
Patients and methods
Patient selection
The survey was conducted in six Italian Oncology Departments with high MPM accrual and expertise (Cliniche Humanitas Gavazzeni, Bergamo; Ospedale S. Spirito, Casale Monferrato; Ospedale SS Antonio e Biagio, Alessandria; Humanitas Cancer Center, Rozzano, Milan; Ospedale Villa Scassi, Sampierdarena, Genova; Istituto Oncologico Veneto, Padova). The clinical records of all elderly patients (⩾70 years old) with MPM diagnosed from January 2005 to November 2011 were reviewed. For each patient, age, asbestos exposure, gender, histology, stage, Eastern Cooperative Oncology Group Performance Status (ECOG-PS), Charlson Comorbidity Index (CCI) (Charlson et al, 1987) and treatment modalities were analysed and correlated to overall survival (OS). Asbestos exposure was evaluated based on information reported in clinical records; exposure was classified as occupational, environmental, uncertain or not documented. Stage was evaluated according to the International Mesothelioma Interest Group (IMIG) staging system (Rusch, 1995). Best tumour response to therapy was assessed according to modified RECIST criteria for MPM (Byrne and Nowak, 2004). Charlson Comorbidity Index was calculated by a Microsoft Excel Macro, available on the web with no restrictions and licence (Hall et al, 2004). An informed consent was obtained by each patient before any diagnostic or therapeutic procedure. All patient data were made fully anonymous.
Statistical analyses
The primary objective of this study was OS, which was calculated as the time from diagnosis until death from any cause; patients who were alive on the date of last follow-up were censored on that date. overall survival was analysed according to the following variables: gender, age (<75 years vs ⩾75 years), ECOG-PS (0 vs ⩾1), histotype (epithelial vs non-epithelial), stage (I–II vs III–IV IMIG stage), asbestos exposure (yes vs no/uncertain), presence of comorbidities (CCI 0 vs ⩾1), and treatment with any pemetrexed-based regimen, including single-agent therapy (yes vs no). Actuarial survival curves were generated using the Kaplan–Meier method (Kaplan and Meier, 1958). Median follow-up time was estimated with the use of the inverse Kaplan–Meier method (Schemper and Smith, 1996). All parameters were evaluated as categorical variables by univariate analysis using the log-rank test to compare groups. For age, the median value of the whole population (75 years) was chosen as cutoff for the analysis. Statistically significant variables were regressed using multivariate Cox proportional hazards model. The effect of each factor was expressed as hazard ratio (HR) with 95% confidence intervals (CIs), considering only statistically significant factors in the multivariable model. Statistical significance was set at P<0.05 for each evaluation. All statistical analyses were performed with the R software package, version 2.0.1 (R Foundation for Statistical Computing, Institute for Statistics and Mathematics, Wien, Austria).
Results
Demographics
Out of a total of 715 consecutive MPM cases diagnosed at the six participating Hospitals in the study period, 241 elderly patients with complete clinical data were identified (34% of the whole population). The patient referral by Institution is reported in Table 1. Of note, the proportion of elderly individuals was similar in all centres. Patient characteristics are listed in Table 2. Median age was 75 years, with a range from 70 to 92. Forty-eight cases (20%) were 80 years or older. Patients were mainly males (64%), with ECOG-PS of 0–1 (94%). Asbestos exposure was documented in 80% of cases; a high rate of environmental exposure was reported, mainly owing to the contribution of two centres (Casale Monferrato and Alessandria, that are located in the same geographical area). Diagnosis of MPM was histological in the vast majority of subjects, with 5% only having a cytological or clinical-radiological definition alone. More than two thirds of patients had an epithelioid histological subtype. IMIG stage was I–II in 149 patients (62%), III–IV in 92 (38%). The details of comorbidity scores according to CCI are reported in Table 3. No major comorbidity (CCI=0) was found in 145 patients (60%), whereas at least one comorbid condition (CCI⩾1) was reported in 92 (38%). Main reported comorbidities were peripheral vascular disease, diabetes and chronic liver disease. In four cases, CCI was unknown.
Table 1. Patient accrual by Centre.
| Institution | Total no. of patients | No. Elderly patients | % Elderly patients |
|---|---|---|---|
| Casale Monferrato/Alessandria Hospitals |
343 |
124 |
36% |
| Humanitas Cancer Center, Rozzano, Milan |
132 |
43 |
33% |
| Genova Sampierdarena Hospital |
95 |
31 |
33% |
| Cliniche Humanitas Gavazzeni, Bergamo |
75 |
25 |
33% |
| Istituto Oncologico Veneto, Padova |
60 |
18 |
30% |
| Total | 715 | 241 | 34% |
Table 2. Patient characteristics (N=241).
| Variable | No. of patients | % of patients |
|---|---|---|
| Age |
Median 75 (range 70–92) |
|
| ⩾75 yrs |
122 |
50% |
| ⩾80 yrs |
48 |
20% |
|
Gender | ||
| Male | 155 | 64% |
| Female |
86 |
36% |
|
Asbestos exposure | ||
| Occupational | 101 | 42% |
| Environmental | 91 | 38% |
| Uncertain | 19 | 8% |
| No documented exposure |
30 |
12% |
|
ECOG performance status | ||
| 0 | 157 | 65% |
| 1 | 69 | 29% |
| 2 | 11 | 4% |
| Unknown |
4 |
2% |
|
Diagnosis | ||
| Histological | 228 | 95% |
| Cytological only | 6 | 2% |
| Clinical-radiological only |
7 |
3% |
|
Histology | ||
| Epithelioid | 165 | 69% |
| Mixed | 27 | 11% |
| Sarcomatoid | 28 | 11% |
| Unspecified/unknowna |
21 |
9% |
|
IMIG stage | ||
| Early (I–II) | 149 | 62% |
| Advanced (III–IV) | 92 | 38% |
Abbreviations: ECOG=Eastern Cooperative Oncology Group; IMIG=International Mesothelioma Interest Group.
Nine patients with histological diagnosis and unspecified subtype; five with cytology only; seven with clinical/radiological diagnosis only.
Table 3. Charlson Comorbidity Index (N=241).
| CCI score | No. of patients | % of patients |
|---|---|---|
| 0 |
145 |
CCI=0 (n=145), 60% |
| 1 |
7 |
CCI⩾1 (n=92), 38% |
| 2 |
11 |
|
| 3 |
2 |
|
| 4 |
39 |
CCI⩾4 (n=72), 30% |
| 5 |
22 |
|
| 6 |
4 |
|
| 7 |
1 |
|
| 8 |
0 |
|
| 9 |
5 |
|
| 10 |
1 |
|
| Unknown | 4 |
Abbreviations: CCI=Charlson Comorbidity Index.
Treatment
Overall, 198 patients (82%) underwent an active treatment, whereas 43 (18%) received best supportive care (BSC) only. Active treatment consisted of multimodality therapy (including any kind of surgery, chemotherapy and in some cases radiotherapy) in 18 and chemotherapy as single modality therapy in 180 patients. In patients ⩾75 years (n=122), 3 cases were treated with multimodality therapy, 86 with chemotherapy, and 33 received BSC. The respective numbers for patients ⩾80 years (n=48) were 0, 30 and 18 cases.
Histological diagnosis in the 18 cases treated with multimodality therapy was first obtained at thoracoscopy; subtype in this subset was epithelioid in 15, mixed in 2, sarcomatoid in 1 case. Pleurodesis was done in nine patients. Pleurodesis alone was not considered a surgical procedure. Most patients (12 cases) underwent pleurectomy/decortication, six had extrapleural pneumonectomy. No perioperative mortality occurred. Patients were selected for surgery based on early stage (I–II), good performance status (ECOG-PS ⩽1), absence of relevant comorbidities (CCI ⩽1) and adequate cardiac and pulmonary function.
First-line chemotherapy (as single treatment or as a part of multimodality therapy) was mainly pemetrexed based, with most patients treated with the combination of pemetrexed and carboplatin (Table 4). Overall, 178 patients (74% of the study population) received a pemetrexed-based regimen. Response was not assessed or not reported in 23 cases; a complete or partial response was achieved in 1 and 45 patients, respectively, for a response rate of 23% 75 patients had stable disease (38%) and 54 progressed; therefore, overall disease control rate was 61%. A second-line treatment was administered to 87 patients (44% of patients treated with first-line chemotherapy, 36% of the whole study group). About a quarter of patients were re-challenged with pemetrexed; the remaining received a gemcitabine or vinorelbine-based regimen, or an experimental agent (Table 4). Overall, response to second-line therapy was observed in six cases (7%), with 26 patients having stable disease (30% overall disease control 37%). In pemetrexed-retreated patients, response rate was 20% (five cases); stable disease was registered in 11 patients (44% overall disease control 64%).
Table 4. Patient treatment.
| Treatment | No. of patients | % of patients |
|---|---|---|
| All |
241 |
|
| Multimodality treatment (MMT) | 18 | 7 |
| Chemotherapy (CT) | 180 | 75 |
| Best supportive care only (BSC) |
43 |
18 |
| First-line chemotherapy |
198 |
|
| Pemetrexed-based chemotherapy | 178 | 90% |
| Pemetrexed single agent | 31 | 16 |
| Pemetrexed+carboplatin | 119 | 60 |
| Pemetrexed+carboplatin+bevacizumaba | 18 | 9 |
| Pemetrexed+cisplatin | 10 | 5 |
|
Other |
20 |
10% |
| Second-line chemotherapy |
87 |
|
| Pemetrexed-based chemotherapyb | 25 | 29 |
| Gemcitabine-based regimenc | 19 | 22 |
| Vinorelbine | 22 | 25 |
| Experimental trial | 21 | 24 |
Treatment was delivered within an experimental trial (Ceresoli et al, 2013).
Including 22 pemetrexed re-challenge, 2 second-line pemetrexed and 1 patient re-challenged with raltitrexed.
Thirteen single agent, six combination therapy (two patients were treated with gemcitabine/vinorelbine).
Survival analysis
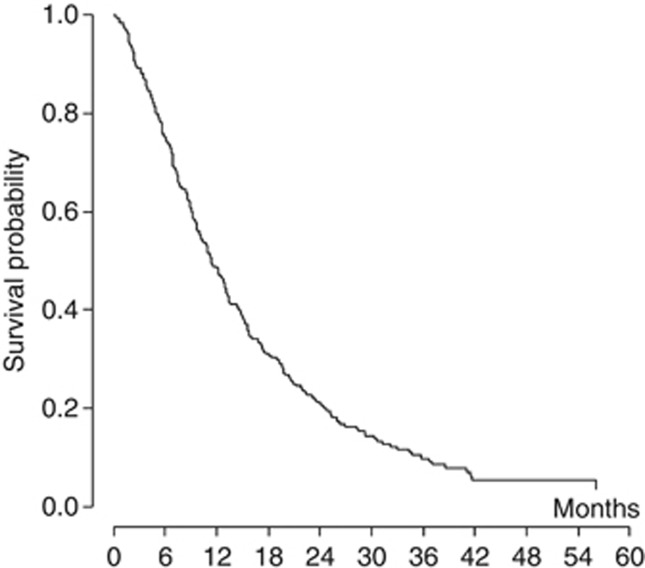
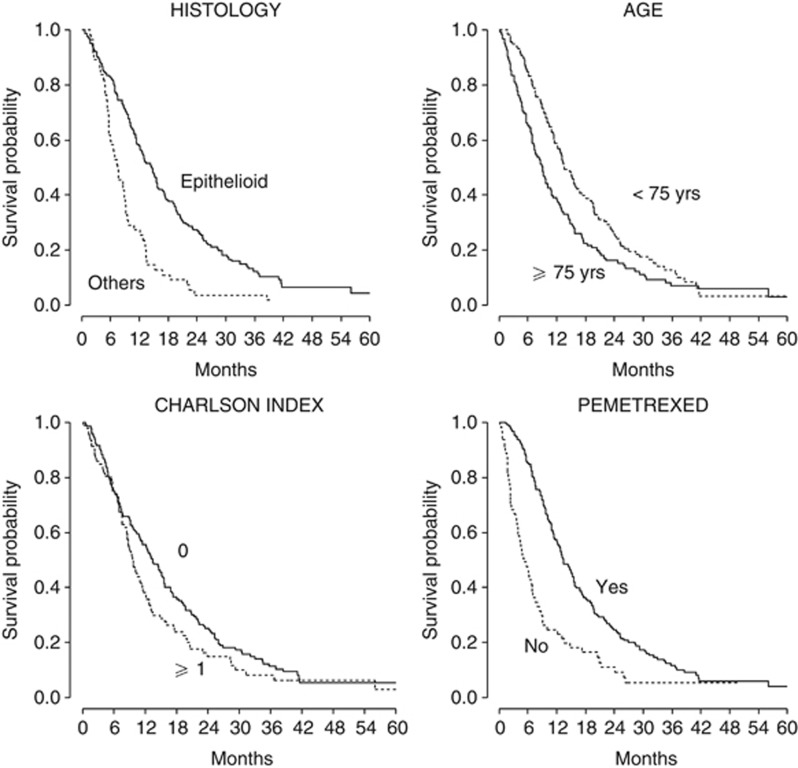
With a median follow-up of 40.1 months (range 0.2–80.8 months), 215 patients died and 26 are still alive. Figure 1 shows the actuarial OS curve for the entire population; the median OS was 11.4 months. The 1-year and 2-year estimates were 48.4% (95% CI: 41.9–54.6%) and 21% (95% CI 16.0–26.5%), respectively. In the univariate model (Table 5), age ⩾75 years, documented asbestos exposure, non-epithelioid histology, advanced (III–IV) IMIG stage, presence of any comorbidity (CCI⩾1) and absence of a pemetrexed-based treatment were significantly correlated to a shorter OS. No survival difference was observed according to gender. Age remained a prognostic factor even when a cutoff of 80 years was considered, with patients⩾80 years showing a median OS of 6.7 months as compared with 13.1 months for patients<80 years (P<0.001). In the multivariate analysis, non-epithelioid histology (HR 2.32; 95% CI 1.66–3.23, P<0.001), age ⩾75 years (HR 1.44; 95% CI 1.08–1.93, P=0.014), advanced IMIG stage (HR 1.47; 95% CI 1.09–1.98, P=0.011), and the presence of comorbidities according to CCI (HR 1.38; 95% CI 1.02–1.85, P=0.034) were confirmed as significantly correlated to a shorter OS. On the contrary, asbestos exposure was not significantly related to OS (HR 1.28; 95% CI 0.89–1.85, P=0.183).
Figure 1.
Kaplan–Meier curve of overall survival for all patients (n=241, median 11.4 months).
Table 5. Prognostic factors analysis (N=241).
|
Univariate analysis | ||
|---|---|---|
| Variable | Median OS (mos) | P-value (univariate) |
| All |
11.4 |
|
|
Gender |
|
|
| Male | 11.4 | 0.460 |
| Female |
10.9 |
|
|
Age (years) |
|
|
| <75 | 13.4 | 0.006 |
| ⩾75 |
8.9 |
|
|
Asbestos exposure |
|
|
| Occupational/ environmental | 10.4 | 0.009 |
| Uncertain/not documented |
15.2 |
|
|
ECOG performance status |
|
|
| 0 | 11.3 | 0.843 |
| ⩾1 |
10.9 |
|
|
IMIG stage |
|
|
| Early (I–II) | 15.2 | 0.021 |
| Advanced (III–IV) |
9.7 |
|
|
Charlson Comorbidity Index |
|
|
| 0 | 13.1 | 0.057 |
| ⩾1 |
9.4 |
|
|
Histology |
|
|
| Epithelioid | 14.5 | <0.001 |
| Non epithelioid |
7.4 |
|
|
Pemetrexed (yes
vs
no) |
|
|
| Yes | 13.2 | <0.001 |
| No | 4.9 | |
|
Multivariate analysis | ||
|---|---|---|
| Variable | HR (CI 95%) | P-value (multivariate) |
| Age |
1.39 (1.04; 1.86) |
0.026 |
| Charlson Comorbidity Index |
1.42 (1.06; 1.91) |
0.019 |
| Histology |
2.40 (1.73; 3.34) |
<0.001 |
| Pemetrexed | 0.41 (0.29; 0.58) | <0.001 |
Abbreviations: CI=confidence interval; ECOG=Eastern Cooperative Oncology Group; HR=hazard ratio; IMIG=International Mesothelioma Interest Group; OS=overall survival.
In the same model, treatment with pemetrexed was associated with improved OS (HR 0.40; 95% CI 0.28–0.56, P<0.001). The OS curves according to significant prognostic factors are shown in Figure 2.
Figure 2.
Kaplan–Meier curves of overall survival stratified for statistically significant prognostic factors (n=241).
Discussion
The incidence of MPM in elderly patients is steadily increasing in Western and industrialised countries. In Italy, mean age at diagnosis, according to the National Mesothelioma Register (Marinaccio et al, 2012) is 69.2 years, with a median latency time of 46 years from first asbestos exposure. Patients older than 65 years represent 67.4% of the registered MPM population, with 26.1% of cases diagnosed between 75 and 84 years. Similar data are reported by other epidemiological studies and mesothelioma registers (Hodgson et al, 2005; Clements et al, 2007; Price and Ware, 2009). Despite these epidemiologic data, retrospective and prospective studies in the elderly population affected by MPM are lacking. We have previously reported the results of a combined analysis of two phase II trials of carboplatin and pemetrexed as first-line therapy in 178 MPM patients (Ceresoli et al, 2008): in that study, pooled data of the two trials were retrospectively analysed for comparison between age groups. Elderly patients were defined as those ⩾70 years. No significant difference was observed in disease-control rate, time to disease progression and survival between elderly patients and their younger counterparts. Toxicity was comparable, with a slightly higher rate of haematological toxicity, mainly cumulative anaemia, in older patients. However, that study had the obvious shortcomings of a post hoc analysis in a group of selected elderly patients (eligible for a combination chemotherapy within a clinical trial); moreover, patients ⩾75 years were poorly represented and data on comorbidities were not available.
In the present study we collected and analysed the clinical data of 241 elderly patients consecutively diagnosed in six centres in nearly a 7-year span after the availability of therapy with pemetrexed. The study population was a true elderly population, as shown by the median age of 75 years, and sufficiently comparable to MPM cases of the Italian National Mesothelioma Register (Marinaccio et al, 2012), even though we had a higher percentage of female patients (36% vs 28%) and epithelioid histology (69% vs 51%). More importantly, the rate of histological diagnosis in our series was very high (95%), whereas in the National Register the rate of confirmed mesothelioma diagnosis was much lower (66.8% in the age cohort between 75 and 84 years and 37.1% in patients of 85 years and older, respectively). Therefore, we cannot exclude a partial selection in the study population by the exclusion of a few cases with dismal prognosis not referred to our centres for diagnosis owing to very advanced age and/or poor ECOG-PS.
Stage was reported as early (I–II according to the IMIG classification) in the majority of our patients. Early stage had a positive prognostic value in multivariate analysis, even though this result should be considered with caution, because of the well-known limitation of IMIG staging system in non-surgical patients (Rusch and Giroux, 2012).
Our analysis confirmed, in the elderly MPM population, the prognostic role of histological subtype, with non-epitheliod tumours carrying a worse prognosis (Curran et al, 1998; Sugarbaker et al, 1999; Ceresoli et al, 2001; Rusch et al, 2012). Age ⩾75 years was also significantly correlated to a shorter OS. In MPM, as in several other cancers, older age has been generally considered a negative prognostic factor (Herndon et al, 1998; Gatta et al, 2006; van der Bij et al, 2012). Apart from age-related biological differences, this may also have been influenced by the lack of specific trials in the elderly population, favouring a nihilistic attitude to treatment (de Magalhães, 2013).
Most elderly patients included in our study had a good ECOG-PS (0–1 in 94% of cases). However, assessment of PS is insufficient to properly address older cancer patients' issues. In recent years, comprehensive geriatric assessment including determination of functional and cognitive status as well as comorbid conditions has emerged as a major tool for evaluation and treatment planning in this setting (Extermann, 2012). In particular, several studies have shown a significant correlation of comorbidity with patient outcome (Brunello et al, 2009; Chen et al, 2012). Different methods of scoring have been proposed, but to date no ideal index has been set as a standard tool (Hall, 2006)Charlson Comorbidity Index is among the most used and validated comorbidity scores in cancer patients (Charlson et al, 1987). It is comprised of a multi-item summative scale with a pre-defined list of weighted items; therefore, it is applicable even in a retrospective setting (Hall et al, 2004). The European Organization for Research and Treatment of Cancer has included CCI in the minimum dataset for the assessment of global health status and functional status in older cancer patients (Pallis et al, 2011). In our series of elderly MPM patients, the presence of any comorbidity evaluated by CCI (i.e., a CCI score ⩾1) was significantly correlated to a shorter OS in multivariate analysis, with a 38% increase of the risk of death.
Treatment with pemetrexed was a strong positive predictive factor for OS. Patients who received any pemetrexed-based chemotherapy survived longer (13.2 vs 4.9 months, P<0.001), with an HR of 0.40. Detailed toxicity data were not available for all patients because of the retrospective nature of the study, and were, therefore, not considered in our analysis. However, treatment with pemetrexed in elderly patients with MPM was generally feasible, confirming previous data in MPM and other cancers (Ceresoli et al, 2008; Gridelli et al, 2012; Gervais et al, 2013; Kim et al, 2013).
In conclusion, in our retrospective study on a large series of elderly MPM patients, non-epithelioid histology, age ⩾75 years, advanced IMIG stage and the presence of comorbidity according to CCI were significant prognostic factors. Although a retrospective non-randomized evaluation of a treatment-related factor should be considered with great caution, therapy with pemetrexed-based chemotherapy was strongly correlated to survival. Based on these data, an invariably nihilistic attitude in MPM elderly patients should be avoided. Prospective dedicated trials in patients selected according to prognostic factors including comorbidity scales are warranted.
The authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
The results of this study have been presented in part at the 48th Annual Meeting of the American Society of Clinical Oncology in Chicago (IL, USA), 1–5 June, 2012 and at the 11th International Mesothelioma Interest Group (IMIG) Conference in Boston (MA, USA), 11–14 September, 2012.
References
- Brunello A, Sandri R, Extermann M. Multidimensional geriatric evaluation for older cancer patients as a clinical and research tool. Cancer Treat Rev. 2009;35:487–492. doi: 10.1016/j.ctrv.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Byrne MJ, Nowak AK. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol. 2004;15:257–260. doi: 10.1093/annonc/mdh059. [DOI] [PubMed] [Google Scholar]
- Castagneto B, Botta M, Aitini E, Spigno F, Degiovanni D, Alabiso O, Serra M, Muzio A, Carbone R, Buosi R, Galbusera V, Piccolini E, Giaretto L, Rebella R, Mencoboni M. Phase II study of pemetrexed in combination with carboplatin in patients with malignant pleural mesothelioma (MPM) Ann Oncol. 2008;19:370–373. doi: 10.1093/annonc/mdm501. [DOI] [PubMed] [Google Scholar]
- Ceresoli GL, Locati LD, Ferreri AJM, Cozzarini C, Passoni P, Melloni G, Zannini P, Bolognesi A, Villa E. Therapeutic outcome according to histologic subtype in 121 patients with malignant pleural mesothelioma. Lung Cancer. 2001;34:279–287. doi: 10.1016/s0169-5002(01)00257-4. [DOI] [PubMed] [Google Scholar]
- Ceresoli GL, Zucali PA, Favaretto AG, Grossi F, Bidoli P, Del Conte G, Ceribelli A, Bearz A, Morenghi E, Cavina R, Marangolo M, Soto-Parra HJ, Santoro A. Phase II study of pemetrexed plus carboplatin in malignant pleural mesothelioma. J Clin Oncol. 2006;24:1443–1448. doi: 10.1200/JCO.2005.04.3190. [DOI] [PubMed] [Google Scholar]
- Ceresoli GL, Castagneto B, Zucali PA, Favaretto A, Mencoboni M, Grossi F, Cortinovis D, Del Conte G, Ceribelli A, Bearz A, salamina S, De Vincenzo F, Cappuzzo F, Marangolo M, Torri V, Santoro A. Pemetrexed plus carboplatin in elderly patients with malignant pleural mesothelioma: combined analysis of two phase II trials. Br J Cancer. 2008;99:51–56. doi: 10.1038/sj.bjc.6604442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceresoli GL, Zucali PA, Mencoboni M, Botta M, Grossi F, Cortinovis D, Zilembo N, Ripa C, Tiseo M, Favaretto AG, Soto-Parra H, De Vincenzo F, Bruzzone A, Lorenzi E, Gianoncelli L, Ercoli B, Giordano L, Santoro A. Phase II study of pemetrexed and carboplatin plus bevacizumab as first-line therapy in malignant pleural mesothelioma. Br J Cancer. 2013;109:552–558. doi: 10.1038/bjc.2013.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Chen RC, Royce TJ, Extermann M, Reeve BB. Impact of age and comorbidity on treatment and outcomes in elderly cancer patients. Semin Radiat Oncol. 2012;22:265–271. doi: 10.1016/j.semradonc.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Clements M, Berry G, Shi J, Ware S, Yates D, Johnson A. Projected mesothelioma incidence in men in New South Wales. Occup Environ Med. 2007;64:747–752. doi: 10.1136/oem.2006.031823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran D, Sahmoud T, Therasse P, van Meerbeeck J, Postmus PE, Giaccone G. Prognostic factors in patients with pleural mesothelioma: the European Organization for Research and Treatment of Cancer experience. J Clin Oncol. 1998;16:145–152. doi: 10.1200/JCO.1998.16.1.145. [DOI] [PubMed] [Google Scholar]
- Dale DC. Poor prognosis in elderly patients with cancer: the role of bias and under-treatment. J Support Oncol. 2003;1 (Suppl 2):11–17. [PubMed] [Google Scholar]
- de Magalhães JP. How ageing processes influence cancer. Nature Rev Cancer. 2013;13:357–365. doi: 10.1038/nrc3497. [DOI] [PubMed] [Google Scholar]
- Delgermaa V, Takahashi K, Park EK, Le GV, Hara T, Sorahan T. Global mesothelioma deaths reported to the World Health Organization between 1994 and 2008. Bull World Health Organ. 2011;89:716–724. doi: 10.2471/BLT.11.086678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diandini R, Takahashi K, Park EK, Jiang Y, Movahed M, Le GV, Lee LJ, Delgermaa V, Kim R. Potential years of life lost (PYLL) caused by asbestos-related diseases in the world. Am J Ind Med. 2013;56:993–1000. doi: 10.1002/ajim.22206. [DOI] [PubMed] [Google Scholar]
- Extermann M. Integrating a geriatric evaluation in the clinical setting. Semin Radiat Oncol. 2012;22:272–276. doi: 10.1016/j.semradonc.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Gatta G, Ciccolallo L, Kunkler I, Capocaccia R, Berrino F, Coleman MP, De Angelis R, Faivre J, Lutz JM, Martinez C, Moller T, Sankila R, and the EUROCARE Working Group Survival from rare cancer in adults: a population-based study. Lancet Oncol. 2006;7:132–140. doi: 10.1016/S1470-2045(05)70471-X. [DOI] [PubMed] [Google Scholar]
- Gervais R, Robinet G, Clément-Duchêne C, Denis F, El Kouri C, Martin P, Chouaki N, Bourayou N, Morère JF. Pemetrexed and carboplatin, an active option in first-line treatment of elderly patients with advanced non-small cell lung cancer (NSCLC): a phase II trial. Lung Cancer. 2013;80:185–190. doi: 10.1016/j.lungcan.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Gridelli C, Brodowicz T, Langer CJ, Peterson P, Islam M, Guba SC, Moore P, Visseren-Grul CM, Scagliotti G. Pemetrexed therapy in elderly patients with good performance status: analysis of two phase III trials of patients with non-squamous non-small-cell lung cancer. Clin Lung Cancer. 2012;13:340–346. doi: 10.1016/j.cllc.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Hall WH, Ramachandran R, Narayan S, Jani AB, Vijayakumar S. An electronic application for rapidly calculating Charlson comorbidity score. BMC Cancer. 2004;4:94–101. doi: 10.1186/1471-2407-4-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SF. A user's guide to selecting a comorbidity index for clinical research. J Clin Epidemiol. 2006;59:849–855. doi: 10.1016/j.jclinepi.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Hamaker ME, Jonker JM, de Rooij SE, Vos AG, Smorenburg CH, van Munster BC. Frailty screening methods for predicting outcome of a comprehensive geriatric assessment in elderly patients with cancer: a systematic review. Lancet Oncol. 2012;13:e437–e444. doi: 10.1016/S1470-2045(12)70259-0. [DOI] [PubMed] [Google Scholar]
- Herndon JE, Green MR, Chahinian AP, Corson JM, Suzuki Y, Vogelzang NJ. Factors predictive of survival among 337 patients with mesothelioma treated between 1984 and 1994 by the Cancer and Leukemia Group B. Chest. 1998;113:723–731. doi: 10.1378/chest.113.3.723. [DOI] [PubMed] [Google Scholar]
- Hodgson JT, McElvenny DM, Darnton AJ, Price MJ, Peto J. The expected burden of mesothelioma mortality in Great Britain from 2002 to 2050. Br J Cancer. 2005;92:587–593. doi: 10.1038/sj.bjc.6602307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Nonparametric estimation of incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- Kim YH, Hirabayashi M, Kosaka S, Nikaidoh J, Yamamoto Y, Shimada M, Toyazaki T, Nagai H, Sakamori Y, Mishima M. Phase II study of pemetrexed as first-line treatment in elderly (⩾75) non-squamous non-small-cell lung cancer: Kyoto Thoracic Oncology Research Group Trial 0901. Cancer Chemother Pharmacol. 2013;71:1445–1451. doi: 10.1007/s00280-013-2142-9. [DOI] [PubMed] [Google Scholar]
- Lichtman SM, Balducci L, Aapro M. Geriatric oncology: a field coming of age. J Clin Oncol. 2007;25:1821–1823. doi: 10.1200/JCO.2007.10.6567. [DOI] [PubMed] [Google Scholar]
- Magnani C, Fubini B, Mirabelli D, Bertazzi PA, Bianchi C, Chellini E, Gennaro V, Marinaccio A, Menegozzo M, Merler E, Merletti F, Musti M, Pira E, Romanelli A, Terracini B, Zona A. Pleural mesothelioma: epidemiological and public health issues. Report from the Second Italian Consensus Conference on Pleural Mesothelioma. Med Lav. 2013;104:191–202. [PubMed] [Google Scholar]
- Marinaccio A, Binazzi A, Marzio DD, Scarselli A, Verardo M, Mirabelli D, Gennaro V, Mensi C, Riboldi L, Merler E, Zotti RD, Romanelli A, Chellini E, Silvestri S, Pascucci C, Romeo E, Menegozzo S, Musti M, Cavone D, Cauzillo G, Tumino R, Nicita C, Melis M, Iavicoli S, ReNaM Working Group. Pleural malignant mesothelioma epidemic: incidence, modalities of asbestos exposure and occupations involved from the Italian National Register. Int J Cancer. 2012;130:2146–2154. doi: 10.1002/ijc.26229. [DOI] [PubMed] [Google Scholar]
- Myojin T, Azuma K, Okumura J, Uchiyama I. Future trends of mesothelioma mortality in Japan based on a risk function. Ind Health. 2012;50:197–204. doi: 10.2486/indhealth.ms1184. [DOI] [PubMed] [Google Scholar]
- Pallis AG, Ring A, Fortpied C, Penninckx B, Van Nes MC, Wedding U, Vonminckwitz G, Johnson CD, Wyld L, Timmer-Bonte A, Bonnetain F, Repetto L, Aapro M, Luciani A, Wildiers H, European Organisation for Research and Treatment of Cancer Elderly Task Force EORTC workshop on clinical trial methodology in older individuals with a diagnosis of solid tumours. Ann Oncol. 2011;22:1922–1926. doi: 10.1093/annonc/mdq687. [DOI] [PubMed] [Google Scholar]
- Park EK, Takahashi K, Hoshuyama T, Cheng TJ, Delgermaa V, Le GV, Sorahan T. Global magnitude of reported and unreported mesothelioma. Environ Health Perspect. 2011;119:514–518. doi: 10.1289/ehp.1002845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price B, Ware A. Time trend of mesothelioma incidence in the United States and projection of future cases: an update based on SEER data for 1973 through 2005. Crit Rev Toxicol. 2009;39:576–588. doi: 10.1080/10408440903044928. [DOI] [PubMed] [Google Scholar]
- Robinson BM. Malignant pleural mesothelioma: an epidemiological perspective. Ann Cardiothorac Surg. 2012;1:491–496. doi: 10.3978/j.issn.2225-319X.2012.11.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch VW. A proposed new international TNM staging system for malignant pleural mesothelioma. From the International Mesothelioma Interest Group. Chest. 1995;108:1122–1128. doi: 10.1378/chest.108.4.1122. [DOI] [PubMed] [Google Scholar]
- Rusch VW, Giroux D. Do we need a revised staging system for malignant pleural mesothelioma? Analysis of the IASLC database. Ann Cardiothorac Surg. 2012;1:438–448. doi: 10.3978/j.issn.2225-319X.2012.11.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch VW, Giroux D, Kennedy C, Ruffini E, Cangir AK, Rice D, Pass H, Asamura H, Waller D, Edwards J, Weder W, Hoffmann H, van Meerbeeck JP, IASLC Staging Committee Initial analysis of the international association for the study of lung cancer mesothelioma database. J Thorac Oncol. 2012;7:1631–1639. doi: 10.1097/JTO.0b013e31826915f1. [DOI] [PubMed] [Google Scholar]
- Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- Sugarbaker DJ, Flores RM, Jaklitsch MT, Richards WG, Strauss GM, Corson JM, DeCamp MM, Jr, Swanson SJ, Bueno R, Lukanich JM, Baldini EH, Mentzer SJ. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg. 1999;117:54–65. doi: 10.1016/s0022-5223(99)70469-1. [DOI] [PubMed] [Google Scholar]
- van der Bij S, Koffijberg H, Burgers JA, Baas P, van de Vijver MJ, de Mol BA, Moons KG. Prognosis and prognostic factors of patients with mesothelioma: a population-based study. Br J Cancer. 2012;107:161–164. doi: 10.1038/bjc.2012.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, Gatzmeier U, Boyer M, Emri S, Manegold C, Niyikiza C, Paoletti P. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]