FIGURE 2.
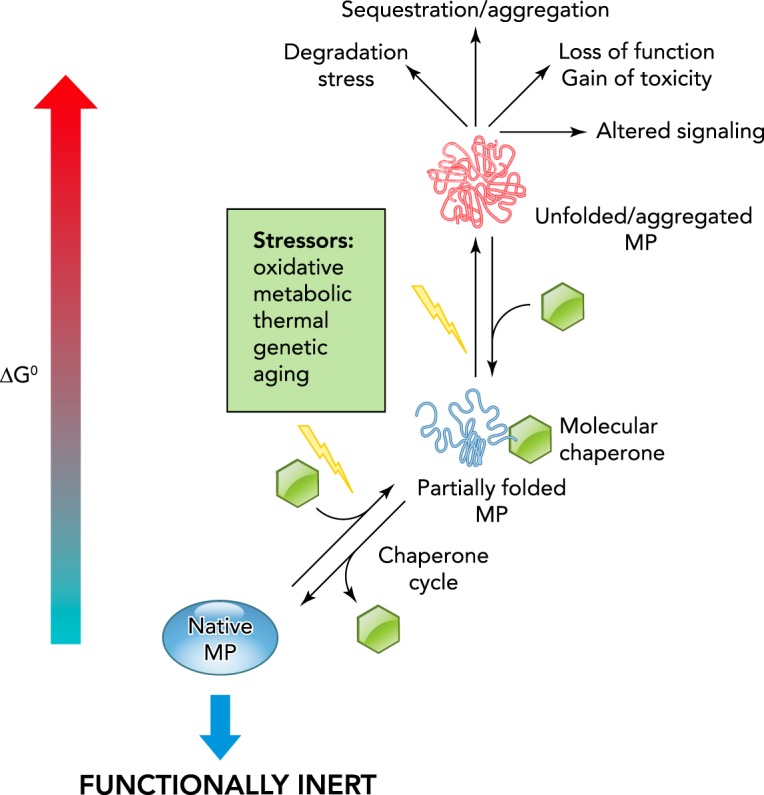
Protein folding and misfolding dynamics in the cellular environment
Multiple external and internal factors (e.g., molecular and chemical chaperones abundance and activity, ion composition, metabolic state, thermal and oxidative stress) can influence the conformational dynamics of PM proteins by shifting their folding-unfolding equilibrium. The energetically favorable conformers are represented by lower folding free energy (ΔG0) of the native polypeptides. Proteotoxic stress- and disease-causing mutations can either thermodynamically and/or kinetically interfere with the folding pathways, as also indicated by the increased ΔG0 of partially folded and unfolded conformers.
