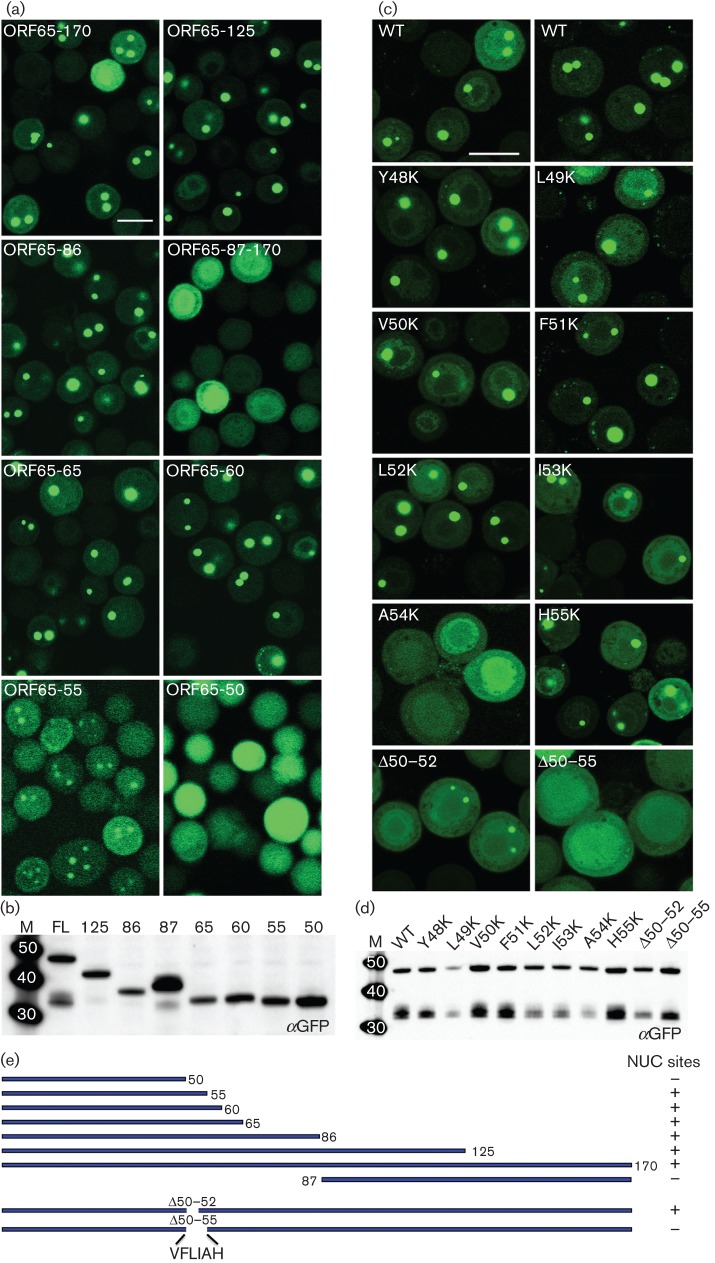
Fig. 6.
Identification of a discrete domain in ORF65 required for interaction with MCP; the amino acid position at A54 is important for this interaction. (a) The region between aa 50 and 60 of ORF65 is required for efficient relocalization of GFP to the nuclear assembly sites. Sf21 cells were co-infected with the vFBD-ORF25/17.5 virus and viruses expressing WT ORF65 (ORF65-170/FL) or truncation polypeptide mutations fused to GFP. The cells were imaged 48 h after infection. Bar, 20 μm. (b) Immunoblot analysis of infected cell lysates with anti-GFP (αGFP) antibody demonstrated stable accumulation of all truncation mutant polypeptides. (c) Substitution of A54 with lysine or deletion of this region abolished interaction with the MCP as judged by the absence of fluorescent puncta in the nucleus. Cells were imaged 24 h after co-infection with viruses expressing ORF65CGFP and vFBD-ORF25/17.5. Bar, 20 μm. (d) Western blot analysis (anti-GFP) of lysates of infected cells demonstrated the presence of stable polypeptide accumulation of each ORF65 mutant polypeptide. Molecular mass standards (kDa) are in lane M. (e) Summary of truncation and in-frame deletion mutant ORF65 polypeptide structures and phenotypes in nuclear (NUC) site relocalization.