SUMMARY
The pathogenic fungus Cryptococcus neoformans must overcome multiple stressors to cause disease in its human host. In this study, we report that C. neoformans rapidly and transiently repressed ribosomal protein (RP) transcripts during a transition from 30°C to host-temperature. This repression was accompanied by accelerated mRNA degradation mediated by the major deadenylase, Ccr4, and influenced by the dissociable RNA polymerase II subunit, Rpb4. Destabilization and deadenylation of RP transcripts were impaired in an rpb4Δ mutant, suggesting that Rpb4 may be involved in host-temperature induced Ccr4-mediated decay. Accelerated decay of ER Stress transcripts one hour following a shift to host-temperature was also impaired in the rpb4Δ mutant. In response to host-temperature, Rpb4 moved from the nucleus to the cytoplasm, supporting a role for Rpb4 in coupling transcription and degradation. The PKH signaling pathway was implicated as a regulator of accelerated degradation of the RP transcripts, but not of the ER stress transcripts, revealing a further level of specificity. When transcription and degradation were uncoupled by deletion of Rpb4, growth at host-temperature was impaired and virulence was attenuated. These data suggest that mRNA synthesis and decay are coupled in C. neoformans via Rpb4, and this tight coordination promotes host-temperature adaptation and pathogenicity.
Keywords: gene regulation, post-transcriptional regulation, adaptation
INTRODUCTION
Cryptococcus neoformans is a basidiomycetous fungus found in a variety of environmental niches worldwide. As a pathogen, C. neoformans causes meningoencephalitis in immune compromised individuals, which is estimated to result in over 600,000 deaths annually (Park et al., 2009). In the human host, C. neoformans encounters a multitude of stresses, including changes in pH, nutrients, O2/CO2 concentration, and temperature, which it must overcome in order to cause systemic disease (Brown et al., 2007). The ability to adapt and proliferate at host-temperature is a unique feature of the few invasive fungal species, and represents a critical virulence trait of C. neoformans (Perfect, 2006).
Proper responses to stress involve specific and rapid changes in gene expression. Changes in transcription following exposure to stressors have been a primary focus of the work dedicated to investigating stress adaptation, and many important stress-response pathways have been delineated (Kraus et al., 2004, Chauhan et al., 2003, Serrano et al., 2006, Maeng et al., 2010). Emerging evidence implicates post-transcriptional events, such as changes in stability/degradation rate of transcripts, as key factors in regulating gene expression (Dori-Bachash et al., 2011, Shalem et al., 2011, Paldanius et al., 2012). Changes in decay kinetics of an mRNA can influence immediately its level of expression, expediting stress responsive changes in gene expression critical for stress adaptation without the need for new mRNA synthesis.
A C. neoformans ccr4Δ mutant, lacking the major mRNA deadenylase, is temperature sensitive and severely attenuated in virulence, indicating that Ccr4-dependent mRNA deadenylation is important for adaptation to the mammalian host (Havel et al., 2011, Panepinto et al., 2007). Deadenylation is the first and rate-limiting step in conventional mRNA decay and plays a major role in post-transcriptional regulation (Chen & Shyu, 2011, Wiederhold & Passmore, 2010). Ccr4 lacks a nucleic acid binding domain and therefore requires RNA-binding proteins to confer specificity and physical association with target mRNAs (Doidge et al., 2012, Goldstrohm et al., 2007, Wiederhold & Passmore, 2010). Co-ordination of post-transcriptional regulons is achieved by the interaction of specific RNA-binding proteins with functionally related classes of transcripts in response to specific stress conditions (Bartlam & Yamamoto, 2010, Goldstrohm et al., 2007, Rendl et al., 2008). We have previously demonstrated that Ccr4-dependent degradation of ER stress transcripts is required for the resolution of the ER stress response during host-temperature adaptation (Havel et al., 2011). Microarray analysis of the ccr4Δ mutant during a shift to host-temperature revealed an accumulation of mRNAs encoding ribosomal proteins relative to the wild type (Havel et al., 2011), suggesting that this class of transcripts also undergoes host-temperature induced Ccr4-dependent post-transcriptional regulation. However, the mechanism by which C. neoformans coordinates temperature-dependent changes in mRNA degradation is unknown.
Recent studies in Saccharomyces cerevisiae demonstrate that mRNA synthesis and degradation are coupled via the interaction of nascent transcripts with the Rpb4p/7p heterodimer, a dissociable subunit of the RNA Polymerase II holoenzyme (Shalem et al., 2011, Dori-Bachash et al., 2011, Goler-Baron et al., 2008). Structural analyses have demonstrated that the heterodimer is associated with the core enzyme via contacts between Rpb7 with Rpb1 and Rpb6, and also through Rpb4 contacts with Rpb1 and Rpb2 (Armache et al., 2005, Armache et al., 2003, Bushnell & Kornberg, 2003). It has been proposed that during stress, the Rpb4p/Rbp7p heterodimer dissociates from the polymerase, but remains associated with specific transcripts and travels with them to the cytoplasm, marking them for rapid degradation, (Shalem et al., 2011). Rpb7 is essential in S. cerevisiae, and it has been found to play a role in general decay, both in 3′ to 5′ and 5′ to 3′ direction (Lotan et al., 2007). Rpb7 can interact with the nascent mRNA as it exits the Pol II machinery through an OB fold single-stranded nucleic acid binding domain (Orlicky et al., 2001, Meka et al., 2005). Because the nature of this nucleic acid interaction is non-specific, it is likely that decay of specific functionally related classes of transcripts is determined by other factors. Interestingly, nonessential Rpb4p specifically promotes the degradation of ribosomal protein transcripts following mild heat shock in S. cerevisiae, and associates with 3′ processing factors and decay factors although full composition of the Rpb4-containing mRNA-protein (mRNP) complex has not been defined (Runner et al., 2008, Lotan et al., 2005). In the model yeast it is has been demonstrated that Rpb4 recruitment is necessary for the degradation of these ribosomal protein transcripts, as overexpression of Rpb7 in an rpb4Δ mutant background does not suppress the RP transcript degradation defect (Lotan et al., 2005). Coupling of transcription and degradation, especially during stress, allows for a fast and transient response at the level of mRNA stability (Dori-Bachash et al., 2011). However, the specific mechanism and regulators of Rpb4-mediated degradation are not clear, and it is unknown if the coupling of transcription and degradation by Rpb4 is conserved in other organisms.
Several signaling pathways in C. neoformans influence the ability of this fungus to grow at host-temperature, including calcineurin, Ras1, and PKC pathways (Odom et al., 1997, Alspaugh et al., 2000, Gerik et al., 2008). However, a role for these pathways in post-transcriptional regulation during host-temperature adaptation has yet to be investigated. Interestingly, the S. cerevisiae orthologs of the mammalian 3-phosphoinositide-dependent kinase (Pdk1), Pkh1p and Pkh2p, are involved in mRNA decay and the formation of P-bodies, cytoplasmic mRNP complexes that mediate mRNA degradation and translational repression (Luo et al., 2011). Pkh1p and Pkh2p regulate both protein kinase C (PKC) signaling and yeast protein kinases Ypk1p and Ypk2p in response to altered sphingolipid homeostasis in S. cerevisiae (Luo et al., 2008, Inagaki et al., 1999, Roelants et al., 2004, Sun et al., 2012). The PKH pathway in C. neoformans plays a pivotal role in stress tolerance and virulence (Chabrier-Rosello et al., 2012, Lee et al., 2012). Host-temperature growth is defective in a C. neoformans strain that is null for the Pkh1 ortholog, Pkh2-02, (Chabrier-Rosello et al., 2012, Lee et al., 2012), but a role in mRNA degradation was not investigated.
In the current study, when transcription and degradation were uncoupled by deletion of the C. neoformans RPB4 ortholog, adaptation to host-temperature was impaired. RP transcripts were transiently repressed during host-temperature adaptation in a Ccr4- and Rpb4-dependent manner. Concurrently, the localization of Rpb4 changed in response to temperature stress, resulting in reduced nuclear localization and punctate accumulation in the cytoplasm immediately following a temperature shift, followed by movement back to the nucleus after longer exposure to the stress. Although Rpb4 does not appear to play a role in the stability of ER stress transcripts under unstressed conditions or at the onset of ER stress, the accelerated degradation of these transcripts following peak induction of the ER Stress Response during host-temperature adaptation was Rpb4-dependent, suggesting a role for Rpb4 in the regulation of stress response intensity and duration. Our studies also revealed that enhanced degradation of RP transcripts, but not ER stress transcripts, was dependent on Pkh2-02 signaling, but was independent of the downstream PKC1-MPK1 MAP kinase cascade. Finally, when transcription and degradation were uncoupled by deletion of Rpb4, virulence was attenuated in a mouse model of disseminated cryptococcosis. Together, these data demonstrate that coupling of transcription and mRNA degradation through Rpb4 plays a fundamental role in regulating gene expression and host-temperature adaptation.
RESULTS
RP transcripts undergo accelerated Ccr4-mediated degradation immediately following exposure to host-temperature
In a microarray analyses that compared the wild type (H99) to a ccr4Δ mutant following a shift from 30°C to 37°C, ribosomal protein (RP) transcripts were the largest functionally related class of transcripts upregulated in the ccr4Δ mutant (GEO accession number GSE28592) (Havel et al., 2011). This suggests that RP transcripts may be repressed in a Ccr4-dependent manner following exposure to host-temperature in the wild type.
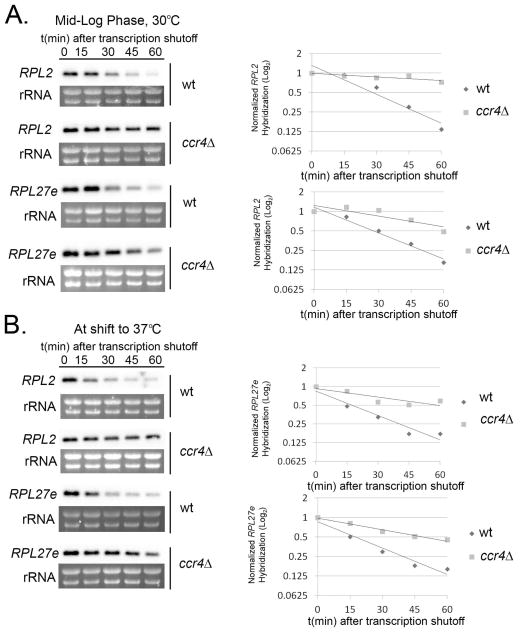
Given the role of Ccr4 in catalyzing the first and rate limiting step in conventional mRNA decay (Wiederhold & Passmore, 2010, Chen & Shyu, 2011), we hypothesized that RP transcripts are destabilized during host-temperature adaptation. To address this hypothesis we analyzed the stability of two representative RP transcripts, RPL2 (CNAG_05232) and RPL27e (CNAG_00779), during 1-hour time courses in which transcription was inhibited with 1,10-phenanthroline while cells remained incubated at 30°C (Fig. 1A) or were shifted to 37°C (Fig. 1B). When wild type cells were incubated under optimal conditions (30°C), the half-lives of RPL2 and RPL27e were 39 and 35 minutes among biological replicates, respectively. When wild type cells were shifted to 37°C, we found that the half-lives of these transcripts were dramatically, and significantly, reduced to 18 minutes (RPL2, p < 0.001) and 19 minutes (RPL27e, p < 0.01). In the ccr4Δ mutant, RP transcripts were stabilized at both temperatures and significantly different compared to wild type (30°C RPL2, RPL27e p < 0.001, p < 0.01; 37°C RPL2 and RPL27e p < 0.001) with half-lives >60 minutes at both temperatures (Fig. 1). These data demonstrate that RP transcripts are immediately destabilized in response to host-temperature, and that the post-transcriptional regulation of RP transcripts requires Ccr4.
Figure 1. Ccr4-mediated degradation of RP transcripts is enhanced following a shift to host-temperature.
Mid-log phase cultures of wild type and ccr4Δ cells were kept at 30°C (A.) or shifted to 37°C (B.) in the presence of the transcriptional inhibitor 1,10-phenanthroline. Aliquots of cells were harvested at 15-minute intervals for RNA extraction and subsequent northern blotting for the detection of RPL2 and RPL27e transcripts. Bands corresponding to transcripts were normalized to rRNA and relative expression was plotted on a log2 graph to determine half-life. Gels and blots shown for RPL2 and RPL27e expression are representative of 3 biological replicates.
Because Ccr4 functions as a poly(A)-specific 3′ → 5′ exonuclease, we next examined poly(A) tail length of RP transcripts using RNase H protection assays (Murray & Schoenberg, 2008). As expected, polyA-tail trimming of RPL2 (Fig. 2) and RPL27e (data not shown) occurs in the wild type as shown by a decrease in size over time. As degradation of RP transcripts is more rapid at host-temperature, it is likely that deadenylation is also more efficient. However, due to the rapid disappearance of RP transcripts at 37°C, it is difficult to observe if polyA-tail trimming occurs more rapidly compared to 30°C. No observable deadenylation occurred at 30°C or at 37°C for either of the RP transcripts in the ccr4Δ mutant, validating the major function of this protein and further supporting that accelerated RP transcript degradation is dependent on Ccr4-mediated deadenylation.
Figure 2. Deadenylation of RP transcripts is absent in a ccr4Δ mutant strain.
RNA extracted from H99 and ccr4Δ during 30° or 37° time courses in the presence of 1,10-phenanthroline were subjected to RNase H digestion to detect polyA-tail length of RPL2 by northern blotting. Oligo dT controls show the length of fully deadenylated RNA.
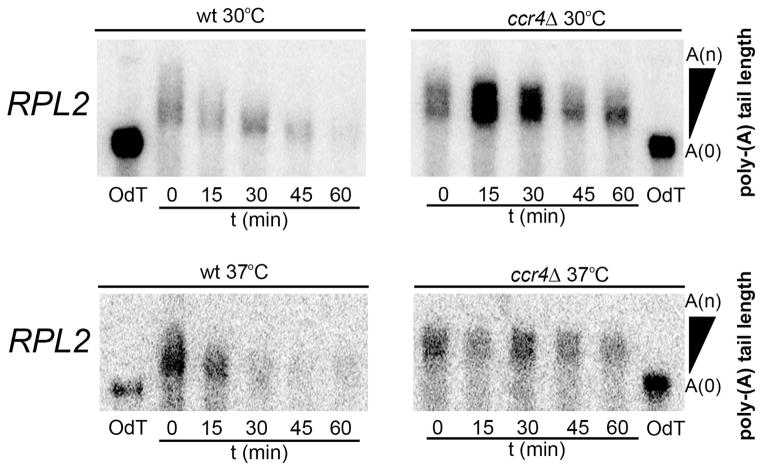
RP transcript repression is a transient response to host temperature
To investigate host-temperature induced effects on overall RP transcript abundance, we analyzed the steady state mRNA levels of RP genes every 30 minutes for 3 hours following a shift to 37°C by northern blot analyses (Fig. 3A and B, top panels). We observed a rapid repression of RPL2 and RPL27e during the first hour following this shift in the wild type. Our results above suggest that enhanced degradation contributes to this repression. Interestingly, during the second hour, levels of these transcripts gradually increase to their pre-shift quantities (Fig. 3C and D, black bars), and degradation kinetics of RP transcripts during this second hour are not statistically different than kinetics during optimal conditions (RPL2 30°C compared to 2 hours post-shift to 37°C, p > 0.05; data not shown). This pattern suggests that repression of RP transcripts is an immediate response to host-temperature that is transient, as RP transcripts gradually increase again, perhaps as the cell adapts to the new temperature environment.
Figure 3. RP transcripts undergo transient repression dependent on Ccr4 during host-temperature adaptation.
Wild type (A and B, top panels) or ccr4Δ cultures (A and B, bottom panels) grown to mid-log phase at 30°C were shifted to 37°C for 3 hours. RNA extracted from aliquots harvested every 30 minutes was analyzed for RPL2 and RPL27e expression by northern blot. Mean expression of RPL2 (C.) and RPL27e (D.) from 3 biological replicates are represented in histograms. Error bars depict SEM. E. Cultures of ccr4Δ cells were shifted to 37°C for 6 hours with maintenance of mid-log growth. RNA was extracted from aliquots harvested every hour and analyzed for RPL2 and RPL27e expression by northern blot. The corresponding histogram shows mean expression of RPL2 and RPL27e normalized to ribosomal bands from 3 biological replicates. All blots shown are representative of 3 biological replicates.
We then analyzed the steady state levels of RP transcripts following a shift to host-temperature in the ccr4Δ mutant and found that repression of RPL2 and RPL27e in the ccr4Δ mutant was modest and more gradual compared to the wild type (Fig. 3A and B, bottom, Fig. 3C and D). These results indicate that RP transcript repression during host-temperature adaptation is defective in the absence of Ccr4, consistent with a role for Ccr4-mediated deadenylation-dependent decay in the repression of RP transcripts during temperature adaptation.
We also noticed that RP transcript repression was not transient in the absence of Ccr4, as RP transcript levels did not return to pre-shift quantities by the third hour in the ccr4Δ mutant. To determine if levels eventually increase in the ccr4Δ mutant, we analyzed the steady state levels of RPL2 and RPL27e for 6 hours following a shift to host-temperature (Figure 3E). After 6 hours, RPL2 and RPL27e continue to undergo very modest repression indicating that this response is clearly defective in the ccr4Δ mutant. We hypothesize that maximal repression of the RP transcripts is required for proper stress responses and adaptation prior to cellular recovery, the lack of which may contribute to the host-temperature growth defect in the ccr4Δ mutant.
Coupling of transcription and degradation via Rpb4 promotes RP transcript degradation following a shift to host-temperature
Studies in S. cerevisiae have suggested that the RNA Polymerase II subunit, Rpb4, functions to couple the synthesis and decay of RP transcripts during temperature stress. (Dori-Bachash et al., 2011, Goler-Baron et al., 2008, Shalem et al., 2011) To determine if this reported role of Rpb4 was conserved in C. neoformans, and if so, whether coupling of mRNA synthesis and decay was important for stress adaptation, we performed a BLASTp search in the C. neoformans grubii H99 genome database (Cryptococcus neoformans Sequencing Project, Broad Institute of MIT and Harvard. (http://www.broad.mit.edu)), and identified locus CNAG_01444 as the C. neoformans RPB4 homologue. We then generated an rpb4Δ mutant by replacing the RPB4 gene with the NAT selection marker by homologous recombination of flanking sequences using biolistic gene transformation. To ensure that CNAG_01444 was a functional homolog of S. cerevisiae Rpb4, we created a myc-tagged Rpb4 strain in the rpb4Δ background (Fig. S1 A). Co-immunoprecipitation and LC-MS/MS revealed that Rpb4-myc physically interacts with Rpb1 and Rpb2, two subunits of the RNA polymerase II core, verifying that Rpb4 is part of the transcriptional complex (Fig. S1 B).
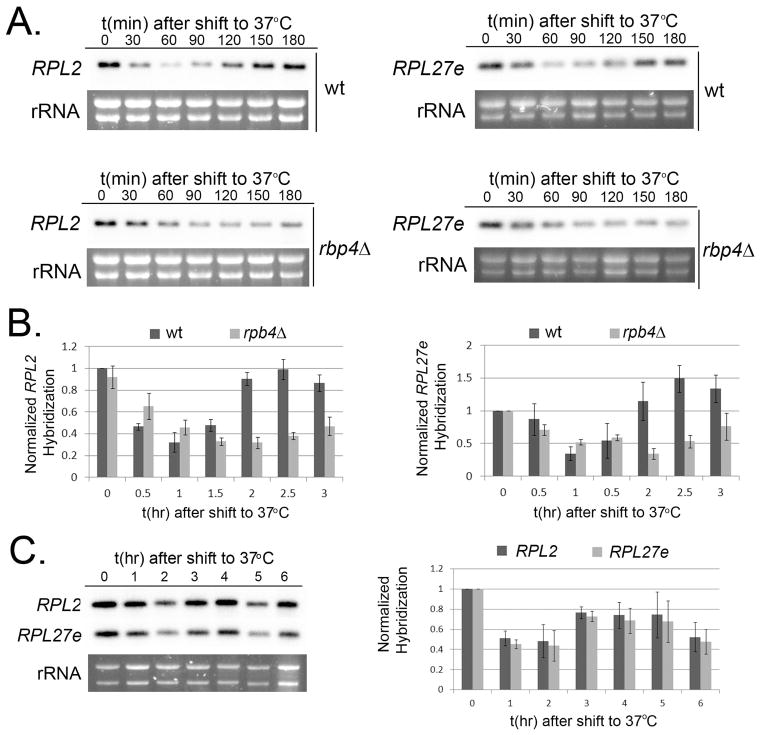
To investigate a role for Rpb4 in the repression of RP-transcripts following a shift to host-temperature, we analyzed the steady state levels of RP genes in the rpb4Δ mutant following a shift to 37°C. Though repression of RP transcripts was observed in this mutant, maximal repression was reached more slowly compared to the wild type, and we only observed a modest subsequent increase in RP transcripts levels by the third hour during this 3-hour time course (Fig. 4A and B). To determine if RP transcripts eventually return to pre-shift quantities at later time points in the absence of Rpb4, we analyzed the steady state levels of RPL2 and RPL27e for 6 hours in the rpb4Δ mutant (Fig 4C). While RP transcripts do begin to increase by 3 hours, expression levels of these mRNAs in the rpb4Δ mutant do not reach those observed in the wild type, suggesting that Rpb4 plays a role in the efficiency of transient RP transcript repression and recovery during host-temperature adaptation.
Figure 4. Rpb4 mediates host temperature induced repression.
A. Mid-log phase cultures of wild type (top) and rpb4Δ (bottom) were shifted to 37°C for 3 hours. RNA extracted from aliquots harvested every 30 minutes was analyzed for RPL2 (left) and RPL27e (right) expression by northern blot. B. Mean expression of RPL2 (left panel) and RPL27e (right panel) from 3 biological replicates. Error bars depict the SEM. C. Mid-log phase cultures of rpb4Δ were shifted to 37°C for 6 hours. RNA extracted from aliquots harvested every 60 minutes was analyzed for RPL2 and RPL27e expression by northern blot. The corresponding histogram represents the mean expression of RPL2 and RPL27e from 3 biological replicates.
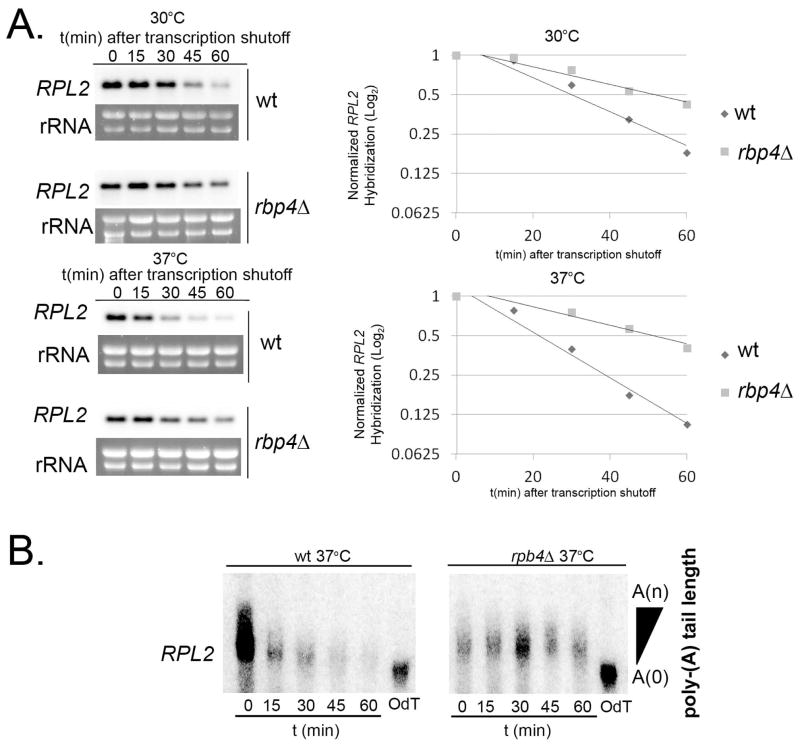
To determine if Rpb4 specifically affects degradation of RP transcripts during host-temperature adaptation we performed 30°C and 37°C stability time-courses with the transcriptional inhibitor 1,10-phenanthroline and analyzed RP transcripts by northern blot. In the rpb4Δ mutant, degradation of RP transcripts was slowed at both 30°C and 37°C and was significantly different compared to wild type (Fig. 5A, 30°C and 37°C, p < .001), although the defect was less severe than that observed in the ccr4Δ mutant. These results indicate that Rpb4 contributes to destabilization of RP transcripts following exposure to host-temperature and suggests that the role of Rpb4 in mRNA degradation is conserved in C. neoformans.
Figure 5. Rpb4 mediates destabilization of RP transcripts at the level of deadenylation.
A. Mid-log phase cultures of H99 and rpb4Δ grown at 30°C were kept at 30°C (top) or shifted to 37°C (bottom) in the presence of 1,10-phenanthroline. RNA extracted from aliquots harvested every 15 minutes was used for northern blot detection of RPL2. Bands corresponding to the transcripts were normalized to rRNA and plotted on log2 graphs to determine half-life. Data presented is representative of 3 biological replicates. B. RNA extracted from H99 and rpb4Δ cultures following a shift to 37°C and transcriptional inhibition was subjected to RNase H degradation to detect polyA-tail length. Oligo-dT controls demonstrate fully deadenylated transcripts.
Rpb4 affects RP transcript degradation at the level of deadenylation
We were interested in determining which step in degradation is affected by Rpb4 upon a shift to host-temperature in C. neoformans. Loss of Rpb4p in S. cerevisiae results in slower deadenylation (Lotan et al., 2005), the first committed and rate limiting step in mRNA degradation. To assess deadenylation in the C. neoformans rpb4Δ mutant, we performed RNase H assays as described above. As demonstrated in Fig. 5B, the poly-A tail lengths of RPL2 transcripts are not changed over the time course in the absence of Rpb4. Lack of poly-A tail trimming in the rpb4Δ mutant is comparable to that in the ccr4Δ mutant indicating that Rpb4 may promote Ccr4-targeted deadenylation during the degradation process.
Rpb4 controls Ccr4-dependent ER stress transcript stability during temperature stress
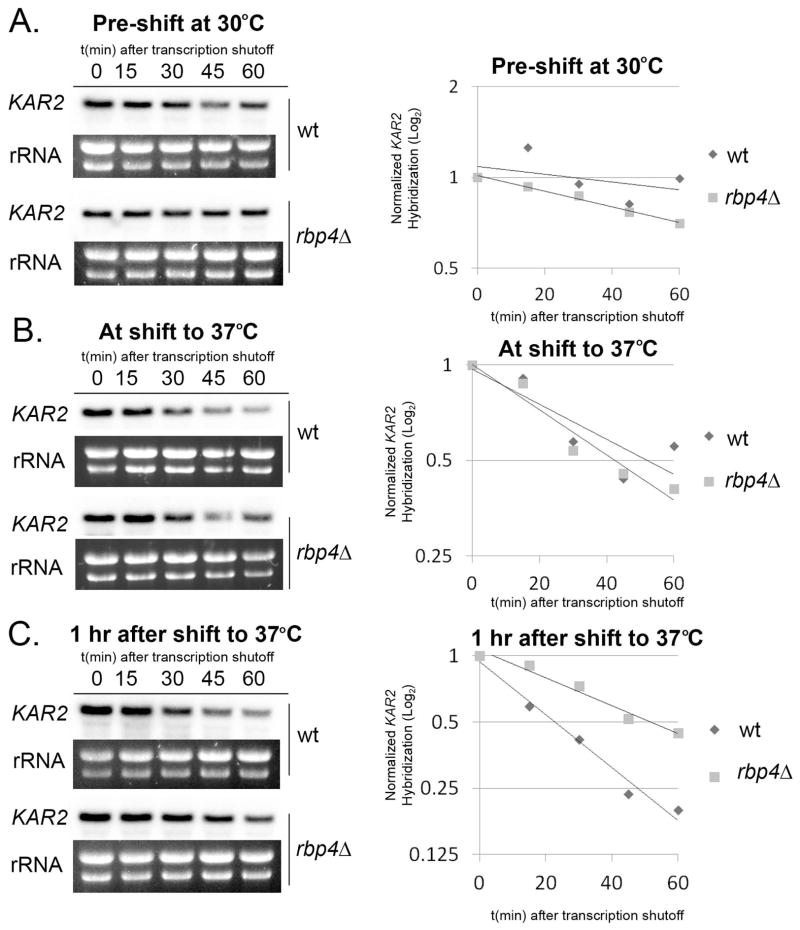
We next asked if Rpb4 is involved in enhanced degradation of other functionally related classes of transcripts during host-temperature adaptation, as Rpb4-mediated degradation has only been demonstrated for RP transcripts in the model yeast. We previously reported Ccr4-dependent degradation of ER stress response transcripts during host-temperature adaptation in C. neoformans (Havel et al., 2011). The steady-state expression pattern of ER stress transcripts is the inverse of RP transcripts, as ER stress transcripts are first induced for one hour immediately following a shift to host-temperature, and then undergo Ccr4-mediated repression to pre-shift levels during the second hour. To determine if Rpb4 also plays a role in the enhanced degradation of ER stress transcripts, we analyzed the stability of the ER stress response transcript KAR2, encoding an ER chaperone, in the rpb4Δ mutant. Interestingly, the stability of KAR2 is unaffected by the absence of Rpb4 during growth at optimal temperature (30°C wt compared to rpb4Δ, p > 0.05) (Fig. 6A). In addition, while KAR2 does undergo destabilization immediately after the shift to 37°C, during induction of the ER stress response, this destabilization appears to be Rpb4-independent (37° wt compared to rpb4Δ, p > 0.05) (Fig. 6B). However, when stability is analyzed beginning one hour following the shift to 37°C, corresponding to the resolution of the ER stress response, accelerated degradation of KAR2 is seen in the wild type strain (wt, 37°C compared to 1 hr post-shift, p < 0.01), but not in the rpb4Δ mutant (wt compared to rpb4Δ 1hr post-shift, p < 0.001) (Fig. 6C). This data suggests that Rpb4 not only couples transcription and degradation, but that this coupling is specific to certain classes of transcripts and is time-dependent. In addition, because KAR2 is a known target of Ccr4 in C. neoformans (Havel et al., 2011), this data supports our hypothesis that Rpb4 promotes Ccr4-targeted degradation.
Figure 6. Rpb4 mediates the temporally controlled destabilization of ER Stress transcript KAR2.
RNA extracted from wild type and rpb4Δ cultures during 1-hour timecourses of transcriptional arrest at 30°C (A.), immediately following a shift to 37°C (B.), or one hour after a shift to 37°C (C.) was analyzed for the expression of KAR2 by northern blot. KAR2 expression was normalized to rRNA and plotted on log2 graphs. All data presented is representative of 3 biological replicates.
Rpb4 accumulates in the cytoplasm during temperature stress
Rpb4 is a dissociable subunit of RNA polymerase II and has been shown to localize in both the nucleus and in the cytoplasm in S. cerevisiae (Farago et al., 2003, Lotan et al., 2005). Based on our findings that Rpb4 plays a role in temperature induced mRNA degradation, we predicted that Rpb4 would localize to the cytoplasm during a shift to host-temperature. To determine and compare the temperature-dependent localization of Rpb4, we performed immunofluorescence microscopy with an Rpb4-myc-tagged strain. To ensure that the myc-tag did not interfere with the function of Rpb4, we performed stability assays and demonstrated that the Rpb4myc strain is able to rescue the defect in destabilization that is seen in the parental rbp4Δ strain (Fig. S1 C).
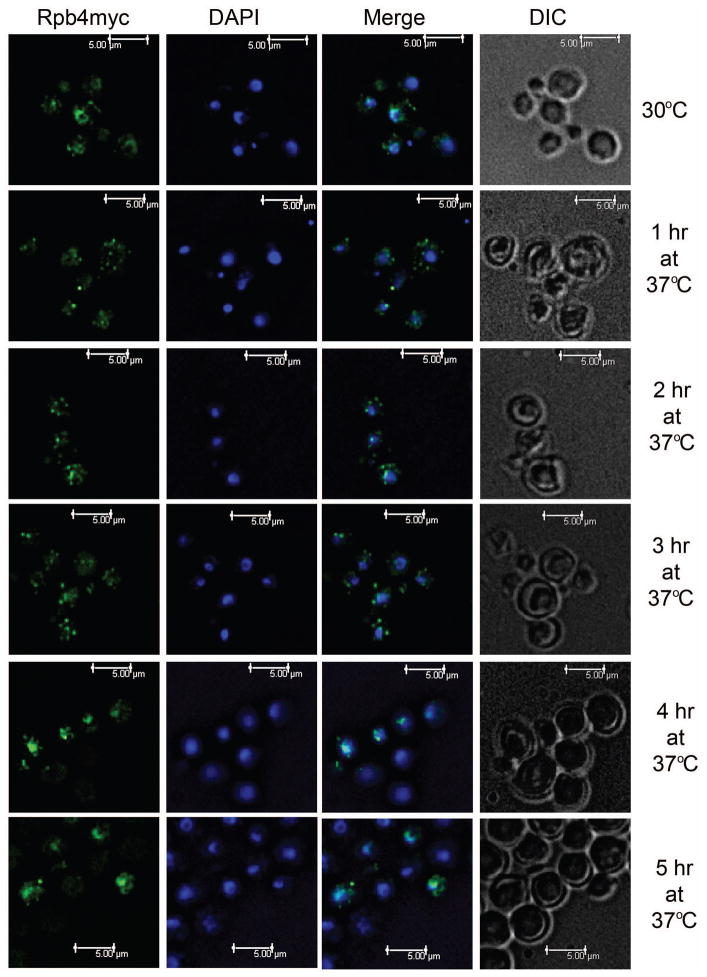
Mid-log phase cells grown at 30°C or shifted to 37°C for up to 5 hours were formadelhyde-fixed, and incubated with anti-myc antibody followed by incubation with Alexaflour 488. At 30°C, Rpb4 was mostly present in the nucleus (Fig. 7, 30°C) with an average of 81% (± 1%) of Rpb4myc fluorescence overlapping with DAPI (Table 2). Co-localization with the nucleus was supported by co-immunoprecipitation of Rpb4-myc with RNA polymerase II subunits Rpb1 and Rpb2 (Fig. S1 B). Following a shift to 37°C for 1 hour, an average of 46% (± 2.2%) of Rpb4-myc was colocalized with DAPI nuclear stain (Table 2), and we observed Rpb4myc accumulation in the cytoplasm in a punctate pattern (Fig. 7, 1hr). This cytoplasmic localization supports our data that demonstrates a role for Rpb4 in mRNA degradation. After 2 hours and 3 hours following a shift to 37°C, we continue to see punctate cytosolic localization in the majority of cells (Fig. 7, 2 hr and 3 hr). We also noticed an increase in nuclear colocalization with the average cell displaying 61% (± 2.5%) and 65% (± 2.2%) overlap with DAPI, respectively, but was not yet equivalent to pre-shift levels (Table 2). By 4 hours, we observe dominant Rpb4myc colocalization with the nucleus in the majority of cells with an average of 86% (± 1.3%) overlap with DAPI nuclear stain (Fig. 7 and Table 2, 4 hr), indicating that at later time points the majority of Rpb4 is in the nucleus. At 5 hours, we observed similar localization with 85% (± 1.2%) of Rpb4myc fluorescence overlapping with DAPI (Fig. 7 and Table 2, 5 hr). This data is supported by our steady state analyses of the RP transcripts as well as the ER stress transcript KAR2, which demonstrate that transcript levels return to pre-shift quantities by these later time-points, presumably when cells begins to recover.
Figure 7. Rpb4 shuttles from the nucleus at 30°C to the cytoplasm following a shift to 37°C.
Cells from mid-log phase cultures of rpb4Δ::RPB4myc grown at 30°C or shifted to 37°C for up to 5 hours were fixed, and Rpb4myc was detected by indirect immunoflourescence with anti-myc antibody followed by Alexaflour 488-conjugated anti-IgG mouse antibody (Green). Nuclear staining with DAPI is shown in blue.
Rpb4 influences growth at host-temperature and virulence in a mouse model
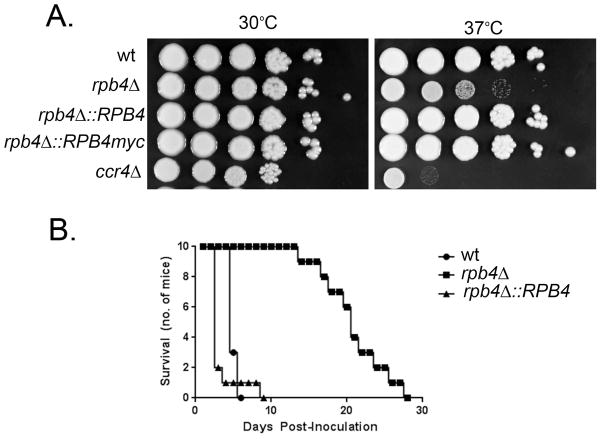
Given that Rpb4 contributes to host-temperature induced stress responses, it was imperative to assess whether Rpb4 has an effect on the ability of C. neoformans to adapt to host-temperature. Growth of the rpb4Δ mutant on spot plates incubated at 30°C was similar to wild type. In contrast, the rpb4Δ mutant exhibited an obvious growth defect at 37°C (Fig. 8A), consistent with a role of Rpb4 in the adaptation of C. neoformans to host-temperature.
Figure 8. Rpb4 influences growth at host-temperature and virulence in a mouse model of disseminated Cryptococcosis.
A. Six 10-fold serial dilutions of wild type, rpb4Δ, rpb4Δ::RPB4, rpb4Δ::RPB4myc, and ccr4Δ were spotted on YPD agar plates and incubated at 30°C or 37°C for 3 days. B. Swiss albino mice were injected with the indicated strain (106 cells) through the lateral tail vein. Morbidity was monitored for 30 days.
Host-temperature adaptation is an essential component of the virulence composite of C. neoformans. To investigate a role for Rpb4 in cryptococcal virulence, we measured the survival of Swiss albino mice following tail vein injection of H99 (wild type), rpb4Δ, rpb4Δ::RPB4, or saline. By day six, 100% of mice injected with H99 and 90% of mice injected with rpb4Δ::RPB4 were sacrificed due to severe head swelling and neurological symptoms (Fig. 8B). Statistical analysis demonstrated no difference between these two groups. Mice injected with rpb4Δ did not begin to succumb to infection until day 14, and symptoms progressed more slowly in these mice. Survival of mice injected with rpb4Δ was significantly longer than those inoculated with either the wild type or rpb4Δ::RPB4 (p < 0.05), indicating that Rpb4 contributes to the pathogenesis of cryptococcosis. Based on our data, we presume that Rpb4 mediates the appropriate reprogramming of mRNA pools to promote adaptation not only to host-temperature but likely to a multitude of stressors encountered in the host environment.
Pkh signaling, independent of the PKC-MPK1 pathway, mediates enhanced degradation of RP transcripts, but not ER stress transcripts during host-temperature adaptation
Several signaling modules that promote host-temperature adaptation have been identified in C. neoformans, though the role of these pathways in mRNA degradation has not been investigated. Using signaling mutants and chemical inhibitors, we assayed acceleration of RP transcript degradation in the cna1Δ, pka1Δ, ras1Δ, pkh2-02Δ, and bck1Δ signaling mutants and in the wild type in the presence and absence of the chemical inhibitor of Tor, rapamycin (Fig. S2, and data not shown). The only difference observed was in the pkh2-02Δ mutant, a strain lacking a protein kinase that is orthologous to mammalian Phosphoinositide-dependent kinase (Liu et al., 2008). Pkh2-02 (Pdk1) in C. neoformans has recently been demonstrated to be critical in cell wall integrity, antifungal resistance, tolerance to oxidative and nitrosative stress, and growth at host temperature (Chabrier-Rosello et al., 2012, Lee et al., 2012).
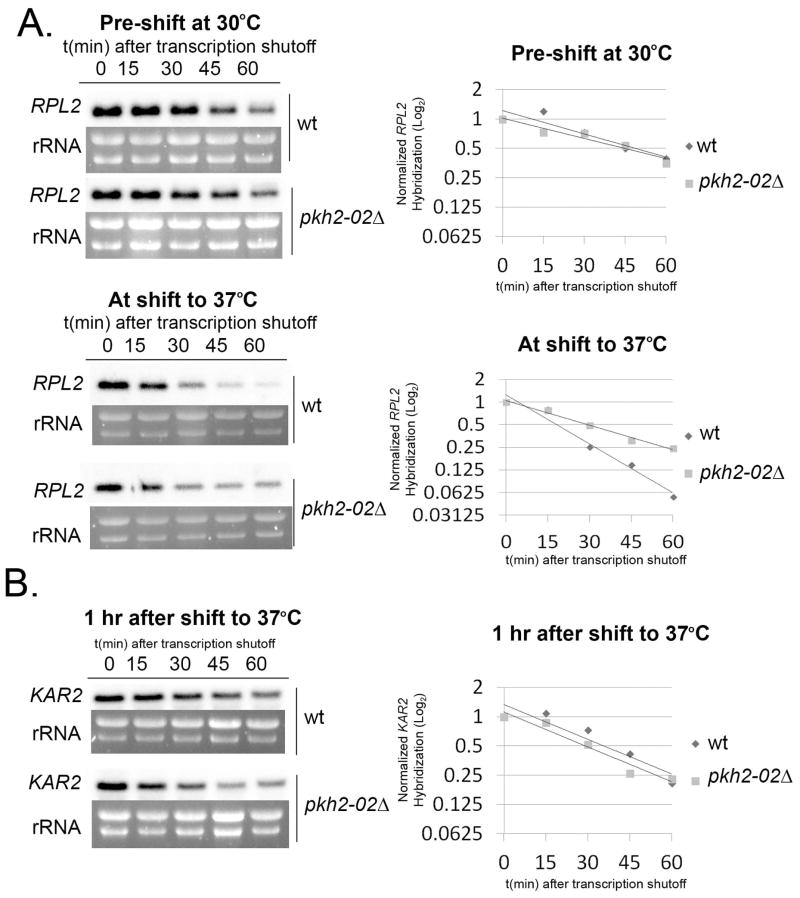
Interestingly, there was no difference in RP transcript degradation between wild type and the pkh2-02Δ mutant at 30°C (p > 0.05, Fig. 9A top panel); however, accelerated degradation that was seen after a shift to 37°C in the wild type was not observed in the pkh2-02Δ mutant (Fig. 9A bottom panel, wt compared to pkh2-02Δ at 37°C, p < 0.001), suggesting that signaling through the PKH pathway is responsible for acceleration of RP transcript degradation during host-temperature adaptation. To ensure that our results were not due to strain differences, we performed these experiments using the parental H99 wild type strain used to produce the pkh2-02Δ mutant (Madhani Collection). Although transcript stabilities were consistently slightly higher in the collection background strain compared to our wild type, the overall patterns observed upon temperature shift were similar.
Figure 9. Signaling through Pkh2-02 mediates destabilization of RP transcripts but not ER stress transcripts during host-temperature adaptation.
A. Wild type H99 from the Madhani Collection (see text) and pkh2-02Δ cultures grown to mid-log phase at 30°C were continued to grow for one hour under transcriptional arrest at 30°C or 37°C. RNA extracted from aliquots during these time-courses was analyzed for RPL2 stability by northern blot. B. Wild type (Madhani Collection) and pkh2-02Δ grown to mid-log phase at 30°C were shifted to 37°C. After one hour, transcription was arrested and aliquots were harvested every 15 minutes for 1 hour. RNA was analyzed for KAR2 stability by northern blot. Expression of all transcripts was normalized to ribosomal RNA and plotted on log2 graphs. All data presented is representative of 3 biological replicates.
Pkh1-dependent activation of Pkc1 influences P-body formation and mRNA degradation during nutrient stress in S. cerevisiae (Luo et al., 2011). To begin investigating the downstream components of the signaling cascade that mediates stress-responsive changes in mRNA degradation, we measured RP transcript decay in the bck1Δ mutant, which is null for the MAPKKK in the PKC-MPK1 signaling pathway. RP transcript decay following a shift to host-temperature in the bck1Δ mutant was similar to wild type (p > 0.05, Fig. S2), suggesting that Pkh2-02 influences host-temperature induced RP transcript degradation independent of the PKC-dependent MAP kinase cascade in C. neoformans.
We were curious to investigate whether Pkh2-02 signaling is also involved in enhanced destabilization of the ER stress transcripts during resolution of the ER stress Response. Interestingly, the pkh2-02Δ mutant did not show a defect in enhanced KAR2 degradation compared to wild type during resolution of the ER stress response, one hour after shift to 37°C (p > 0.05, Fig. 9B). These data suggest that while the RP-transcripts and the ER stress response transcripts undergo Rpb4-dependent degradation during host-temperature adaptation, the enhanced degradation of these two classes of transcripts is not mediated by the same signaling pathway.
DISCUSSION
Host-temperature adaptation in C. neoformans involves a transient repression of ribosomal protein encoding mRNAs that is dependent on mRNA degradation. In the absence of the major deadenylase, Ccr4, these transcripts are stabilized, indicating that Ccr4-targeted poly-A tail trimming is necessary for their degradation. Here we show that, in addition to the specificity of this response, the repression of RP transcripts is transient, lasting only an hour, before levels begin to rise to pre-shift abundance (Fig. 3). Furthermore, the severe growth defect observed with the ccr4Δ mutant at host-temperature suggests that the rapid and transient degradation of RP transcripts may play a pivotal role in host-temperature adaptation.
A similar response to elevated temperature growth in S. cerevisiae has been shown to be mediated by a dissociable subunit of RNA polymerase II, Rpb4, which imprints RP transcripts in the nucleus and targets them for degradation (Herruer et al., 1988, Lotan et al., 2005). Consistent with these previous reports, we found that RP transcripts are stabilized in the absence of Rpb4, that deadenylation of RP transcripts after a shift to 37°C is dependent on Rpb4, and that Rpb4 moves from the nucleus to the cytoplasm following a shift to host-temperature. Taken together, these data demonstrate that the role of Rpb4 is conserved in C. neoformans and suggest that imprinting by Rpb4 is a prerequisite for Ccr4-mediated decay or that Rpb4 may be involved in Ccr4 recruitment. We are currently investigating whether Rpb4 and Ccr4 interact under these conditions to further understand this mechanism.
This coupling of transcription and degradation, mediated by imprinting of RP transcripts by Rpb4, provides an elegant and efficient means to rapidly respond to the changing needs of the cell under stress. However, it was unclear if this role for Rpb4 was specific for RP transcripts, or if Rpb4 mediates the degradation of other classes of transcripts that undergo post-transcriptional regulation under stress conditions. We had previously reported that resolution of the ER stress response during host-temperature adaptation is dependent on Ccr4-mediated degradation of ER stress transcripts (Havel et al., 2011). Intriguingly, we found that in the absence of Rpb4, the enhanced degradation of the ER stress transcripts following their peak induction is lost (Fig. 6C). This is a novel role for Rpb4 and, moreover, demonstrates that Rpb4 mediated mRNA degradation is not exclusive to RP transcripts in C. neoformans. These data also suggest that Rpb4-mediated mRNA degradation may be more subtle and complex than previously thought. While RP-transcripts, which are highly abundant and have high basal levels of transcription, are immediately repressed, the ER stress transcripts do not undergo enhanced degradation until after they reach peak induction, which is observed one hour after exposure to host-temperature (Havel et al., 2011). Thus, it appears that Rpb4 is able to mediate the degradation of different classes of transcripts, at different times, presumably according to different stimuli.
We show that this temporally controlled Rpb4-dependent degradation influences the intensity and duration of both RP transcript repression and ER stress transcript induction in response to host-temperature, ultimately affecting the rapid and transient nature of these events. This is reflected in our in vivo mouse model of disseminated cryptococcosis, which showed that while the wild type very quickly caused disease and morbidity, infection with rpb4Δ cells results in longer duration before the onset of disease and prolonged disease progression (Fig. 8B). It is likely that Rpb4 contributes to the efficiency of reprogramming mRNA pools in response to several stresses encountered in the mammalian host, and impairment in adaptation efficiency by loss of Rpb4 results in attenuation of virulence in this model.
Our localization studies further support the role of Rpb4 in contributing to the transient changes that occur following exposure to host-temperature, and suggest that Rpb4 is acting early to specifically influence host-temperature adaptation, with cytoplasmic localization evident at times when RP transcript decay was affected. Elegant studies have shown that mRNA decay occurs in punctate “processing-bodies”, or P-bodies, in the cytoplasm during stress (Sheth & Parker, 2003, Parker & Sheth, 2007). Our data and data from Rpb4p in S. cerevisiae (Lotan et al., 2005) may suggest that Rpb4 localizes to these P-bodies, and our future studies will directly address this hypothesis. Rpb4 was predominantly located in the nucleus by the fourth hour, presumably to function in the resumption of RP transcript synthesis. Together our data reflects the importance of Rpb4-mediated coupling of transcription and decay in the early stages of host- temperature adaptation.
The responses to external stimuli in C. neoformans, like most eukaryotes, are governed by multiple signal transduction pathways that culminate in the re-programming of gene expression. The focus of these studies in C. neoformans has been the activation of trans-acting factors that regulate mRNA synthesis, and how activation or inactivation of signaling pathways impacts genome-wide steady state mRNA abundance. As part of our study, we asked which of the known signaling pathways in C. neoformans that impact temperature adaptation might be involved in the regulation of Ccr4- and Rpb4-dependent mRNA degradation. To our surprise, unlike what has been reported in S. cerevisiae (Munchel et al., 2011, Albig & Decker, 2001), the acceleration of RP transcript degradation during temperature adaptation was independent of TOR, as treatment with rapamycin had no effect on RP transcript stability during temperature stress. Likewise, we were surprised to find no effect of calcineurin deletion on RP transcript stability given the recently described association of calcineurin with cytoplasmic mRNA processing (P) bodies during temperature stress in C. neoformans (Kozubowski et al., 2011).
Our studies led us to identify the C. neoformans PKH signaling pathway as a regulator of RP transcript degradation, as deletion of PKH2-02 resulted in a loss of accelerated RP transcript decay during temperature stress (Figure 9). Pkh2-02 does not appear to influence unstressed Ccr4- and Rpb4-dependent RP transcript decay, as there was no effect on RP transcript decay rate at 30°C in a pkh2-02Δ mutant. C. neoformans Pkh2-02 is orthologous to mammalian phosphoinositide-dependent kinase (Pdk1), and has recently been shown to have a substantial role in mediating stress tolerance (Chabrier-Rosello et al., 2012, Lee et al., 2012, Liu et al., 2008). Activation of PKH signaling is regulated by sphingolipid homeostasis in S. cerevisiae (Liu et al., 2005). Interestingly, sphingolipids also influence the formation of P-bodies during mild temperature stress in the model yeast, and changes in sphingolipid chemistry that influence signaling are rapid and transient (Cowart et al., 2010). It has also been shown that PKH signaling influences the formation of P-bodies during nutrient stress in S. cerevisiae (Luo et al., 2011). In addition, studies in C. neoformans have shed light on the importance of sphingolipid signaling in pathogenicity (Rittershaus et al., 2006, Luberto et al., 2001, Heung et al., 2004). Because Pkh2-02 signals through Pkc1, Sch9 and Ypk1, further study will determine which of these downstream branches might mediate Rpb4-dependent mRNA decay in C. neoformans.
We went on to investigate if the accelerated degradation of the ER stress sensor KAR2 at the point of its maximal induction was likewise Pkh2-02 dependent, and discovered that loss of Pkh2-02 had no effect on KAR2 decay kinetics. We interpret this result to suggest an additional layer of specificity to Rpb4- and Ccr4-dependent mRNA decay, i.e., the upstream signals that mediate changes in mRNA decay kinetics may, in fact, be specific to each RNA regulon. We have yet to identify the stress response pathways that do promote KAR2 decay during temperature adaptation.
Through our studies of Rpb4- and Ccr4-dependent mRNA degradation, we have uncovered multiple layers of specificity. First, stress induction results in the accelerated decay of specific groups of functionally related mRNAs, or regulons. Second, there is temporal specificity controlling the timing of the acceleration of mRNA decay during the stress response. Finally, the upstream signals that promote accelerated decay are specific to discrete regulons. Despite these results, there are likely additional layers of specificity that we have yet to uncover. For instance, the lack of an RNA binding domain with sequence specificity in Rpb4 suggests that there may be an RNA binding partner that confers target specificity to Rbp4 association. Furthermore, the effectors of Pkh2-02 signaling that promote Rpb4-dependent acceleration of RP transcript mRNA decay are yet unknown, as are specific post-translational modifications that might signal Rpb4 association with mRNAs and the exit of these mRNPs out of the nucleus and into the cytoplasm where mRNA decay can occur. It is possible that Rpb7, the partner of Rpb4, may also have a role in the regulation of mRNA decay. The non-specific nature by which Rpb7 interacts with nucleic acid casts doubt on a role for it as a specificity factor, but this will be investigated in future studies that will attempt to identify the protein components of the degradation-destined Rpb4-containing mRNP.
C. neoformans and C. gattii are the only species of Cryptococcus able to cause deep infection in humans. This is likely due, in part, to evolution of temperature adaptability. Through these studies, we have demonstrated that post-transcriptional regulation of gene expression is important for this process, and that the synthesis of mRNA and its eventual degradation are tightly coordinated through a subunit of the RNA polymerase II, Rpb4. Further studies of the role of mRNA degradation and the factors that mediate its specificity in the host-temperature adaptation process will define the mechanism by which C. neoformans re-programs mRNA pools to promote host adaptation and pathogenesis.
MATERIALS AND METHODS
Production of strains
A C. neoformans rbp4Δ strain was constructed as described previously (Panepinto et al., 2005). The knockout construct was verified by sequencing and PCR amplified using primers 5′RPB4KO-PCR and 3′RPB4KO-PCR (Table S1). Biolistic gene transfer was used to introduce the construct into wild type strain H99 according to standard protocol (Toffaletti et al., 1993). NAT resistant colonies were screened for gene replacement by PCR with primers 5′RPB4KO-screen and 3′RPB4KO-screen (Table S1). PCR-positive clones were further verified by northern and Southern blot.
Complementation of the rpb4Δ strain was achieved by introduction of the RPB4 gene with 1000bp upstream and downstream cloned adjacent to the neomycin resistance cassette (Jennifer Lodge, Washington University) in pJet-NEO to create pJetNEO-RPB4. The construct was linearized with NotI and transformed into rpb4Δ by biolistic gene transfer (Toffaletti et al., 1993). G418 resistant colonies were screened by uncut Southern blot to verify genomic integration and northern blot to verify expression of RPB4.
To generate a myc-tagged RPB4 strain, the plasmid pJetNEO-RPB4 (see above) was subjected to site directed mutagenesis using the QuikChange Site-directed Mutagenesis kit (Agilent) with primers RPB4-myc and RPB4-myc-RC (Table S1) to allow insertion of the myc tag immediately upstream of the stop codon. In frame insertion of the myc tag was verified by sequencing. Plasmid DNA was linearized with NotI, and introduced into the rpb4Δ mutant by biolistic transformation (Toffaletti et al., 1993). G418 resistant colonies were screened by Northern blot analysis for RPB4 expression, Western blot with anti-myc antibody for the detection of myc-tagged Rpb4, and by spot plate analysis at 37°C to ensure that myc-RPB4 rescued the growth phenotype.
A ccr4Δ mutant was described previously (Panepinto et al., 2007). The bck1Δ strain was obtained from the deletion collection of Jennifer Lodge (strain KGCN196) through the fungal genetics stock center (McCluskey et al., 2010). The pkh2-02Δ and the wild type H99 strain in which it was produced are part of the Madhani collection (Liu et al., 2008).
Steady State and Stability Timecourses
Overnight cultures were diluted to OD600=0.2 in YPD and grown to mid-log phase (OD600=0.6) at 30°C, 250 rpm in baffled flasks. Cells were pelleted by centrifugation at 4000 x g for 5 minutes. For steady state analysis of transcripts, cells were resuspended in 40 ml pre-warmed 30°C or 37°C YPD and growth was continued at respective temperatures. Pre-warmed media was added as necessary to maintain mid-log growth. 5 ml aliquots were pelleted by centrifugation every 30 minutes or every hour and stored at −80°C until RNA extraction. For transcript stability analyses, cells were resuspended in 30 ml pre-warmed 30°C or 37°C YPD supplemented with 250 μg ml−1 1,10-phenanthroline and growth was continued at respective temperatures. 5 ml aliquots were harvested every 15 minutes for one hour and stored at −80°C until RNA extraction. For stability of ER stress transcripts after peak induction, cells were harvested and resuspended in pre-warmed 37°C YPD. After one hour of incubation at 37°C, 1–10 phenanthroline was added to cultures (250 μg ml−1) and 5 ml aliquots were harvested every 15 minutes for one hour. For stability of RP transcripts 2 hours following a shift to host-temperature, mid-log phase cells grown under optimal conditions were harvested and resuspended in pre-warmed 37°C YPD. After two hours of incubation at 37°C, 1–10 phenanthroline was added to cultures (250 μg ml−1) and 5 ml aliquots were harvested every 15 minutes for one hour.
RNA extraction and Northern blot analyses
For RNA extraction, cells were lysed by mechanical disruption with glass beads in the presence of 2-mercaptoethanol for 5 minutes, alternating between vortexing and incubation on ice every 30 seconds. Lysate was cleared by centrifugation and RNA was extracted using the Qiagen RNeasy Kit. For northern blot analyses (stability and steady state) 3 μg of RNA was electrophoresed through formaldehyde-agarose gel, transferred to nylon membrane, crosslinked and hybridized with P32-labeled DNA probes. After washing, blots were exposed to phosphor screen, and imaged on a Bio-Rad Personal Molecular FX System. Hybridization signal was quantitated using Quantity One software and normalized to the fluorescence signal of the ribosomal bands. Statistical analyses for stability data was obtained by determining the least squares fit of one-phase exponential decay nonlinear regression with Graphpad Prism software. Significance between curves was detected by sum-of-squares F test, with p < 0.05 determining that the data fall on separate regression lines, and therefore exhibit different rates of decay.
RNaseH Assay
RNaseH assays were performed using RNA extracted from stability timecourses. 5 μg of RNA was added to 10 μL of 5x RNaseH buffer (400 mM KCl, 250 mM Tris-HCl (pH 7.5), 50 mM MgCL2, 5 mM DTT), 2.5 μL of cleavage primer (10 μM) (Table S1), and RNase-free water to 46 μL in an eppendorf tube. Tubes were incubated in a 95°C heat block for 5 minutes, immediately transferred to a 42°C water bath and allowed to cool to 32°C on the bench top. 2.5 μL of RNaseOut (invitrogen) and 0.75 μL of RNaseH (Ambion) were added to reactions and tubes were incubated at 37°C for 90 minutes. 2.5μL of 0.5 M EDTA was added to reactions and RNA was ethanol precipitated. RNA was resuspended in 1x TBE-urea sample dye, loaded onto a 10% TBE-urea polyacrylamide gel, and electrophoresed at 190V. The gel was transferred to Nylon membrane and probed with a short P32-labeled RPL8 DNA probe encoding the last 200bp of the transcript. Blots were exposed to a phosphor screen and phosphor-imaged.
Immunoflourescence microscopy
To detect localization of Rpb4, H99 (control for autoflourescence) and the Rpb4-myc complemented rpb4Δ strain were grown to mid-log phase in YPD, 250 rpm, 30°C. A 5 ml aliquot of cells was pelleted by centrifugation at 4,000 x g for 5 minutes and fixed with 3.7% formaldehyde for 20 minutes at room temperature. The remaining culture was harvested by centrifugation and resuspended in pre-warmed 37°C YPD and incubated at 37°C. Aliquots of cells were pelleted every hour for 5 hours and fixed as above. Pre-warmed YPD was added as necessary to maintain mid-log growth. Fixed cells were washed with PBS and resuspended in spheroplast buffer (64 μg/mL “Vinoflow” in 20 mM Sodium citrate, 1M sorbitol, pH 5.4) and incubated at 30°C for 45 minutes. Cells were washed with PBS then resuspended in 1:500 mouse anti-myc antibody in PBS. Cells were incubated at 4°C, overnight, with rotation. Cells were washed twice with PBS, then resuspended and incubated in PBS + anti-mouse IgG Alexafluor 488 secondary antibody (1 μg ml−1) for one hour at room temperature with rotation and shielded from light. Cells were washed, resuspended in PBS, and spotted onto Histogrip (Invitrogen) treated slides. Slides were mounted with Prolong gold Antifade reagent (Invitrogen). Z-stack images of cells were captured using a Leica AM TIRF MC-fluorescence microscope and images were deconvolved by the blind method deconvolution algorithm using LAS AF software (Leica). To determine Rpb4myc colocalization with the nucleus, 87–95 cells (n) from 5–7 fields of view were analyzed using Fiji Colocalization Software to determine the Mander’s coefficient, equating to the proportion of GFP (Rpb4myc) fluorescence that overlapped with DAPI. For statistical analyses, an arcsin-square root transformation was applied to the percentage of overlap values followed by one-way ANOVA. The mean overlap values for all time points were compared to optimal conditions (30°C) using Dunnett’s multiple comparison test post hoc. A p-value less than 0.05 was deemed significant.
Spot plate analyses
For spot plate analyses, overnight cultures of H99, rpb4Δ, ccr4Δ, and rpb4Δ::RPB4 in YPD were washed twice with water, diluted to OD600=1.0, and serially diluted 10-fold five times. 5 μL of each serial dilution was spotted onto YPD agar plates supplemented with indicated reagents. Plates were incubated at indicated temperatures for 3 days.
In vivo virulence analyses
A mouse model of disseminated Cryptococcosis was utilized to compare the virulence of H99 and rpb4Δ. Overnight cultures of H99, rpb4Δ, and RPB4:rpb4Δ were harvested and washed twice with sterile PBS. Cells were counted using a hemacytometer and diluted to 1×107 ml−1 in PBS. For each strain tested, ten 6 week old female NIH Swiss albino mice were injected via tail vein with 100 μl of cells (1×106 cells) or 100 μL of PBS for saline controls. Mice were monitored twice daily until ruffling of fur was observed. Upon ruffling, monitoring was increased to six times daily. Mice that displayed neurological symptoms and/or appeared moribund were sacrificed in a CO2 chamber followed by cervical dislocation. Protocols used for animal studies were approved by IACUC at SUNY Buffalo. Statistical analyses were calculated using the Kruskall-Wallis analysis (ANOVA on Ranks). Pairwise analysis was performed using Dunn’s test post-hoc using GraphPad Prism Software.
Supplementary Material
Table 1.
Colocalization of Rpb4myc with DAPI following shift to host-temperature.
| Time (hr) after shift to 37°C | n (no. of cells) | Mean Percent Rpb4myc-DAPI Co-localization | SEM | p value (vs. t=0) |
|---|---|---|---|---|
| 0 | 89 | 81.1% | ± 1.0% | N/A |
| 1 | 87 | 46.6% | ± 2.2% | <.0001 |
| 2 | 83 | 61.4% | ± 2.5% | <.0001 |
| 3 | 95 | 65.2% | ± 2.1% | <.0001 |
| 4 | 89 | 86.2% | ± 1.3% | ns |
| 5 | 90 | 85.3% | ± 1.2% | ns |
Acknowledgments
We are grateful to Damian J. Krysan, Jennifer Lodge, Hiten Madhani and the Fungal Genetics Stock Center for the construction and dissemination of strains used in this study. We thank Laurie K. Read for critical reading of the manuscript, and the Read Lab for consultation on the technical aspects of the biochemical analyses presented herein.
This work was supported, in part, by United States Public Health Service grants K22 AI070647 and 1R01 AI089920-01A1 to JCP, the University at Buffalo School of Medicine and Biomedical Sciences and the NY State Center of Excellence in Bioinformatics and Life Sciences. ALMB was also supported by the T32 AI007614 training grant to the Witebsky Center for Microbial Pathogenesis and Immunology.
Literature Cited
- Albig AR, Decker CJ. The target of rapamycin signaling pathway regulates mRNA turnover in the yeast Saccharomyces cerevisiae. Molecular biology of the cell. 2001;12:3428–3438. doi: 10.1091/mbc.12.11.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alspaugh JA, Cavallo LM, Perfect JR, Heitman J. RAS1 regulates filamentation, mating and growth at high temperature of Cryptococcus neoformans. Molecular microbiology. 2000;36:352–365. doi: 10.1046/j.1365-2958.2000.01852.x. [DOI] [PubMed] [Google Scholar]
- Armache KJ, Kettenberger H, Cramer P. Architecture of initiation-competent 12-subunit RNA polymerase II. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:6964–6968. doi: 10.1073/pnas.1030608100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armache KJ, Mitterweger S, Meinhart A, Cramer P. Structures of complete RNA polymerase II and its subcomplex, Rpb4/7. The Journal of biological chemistry. 2005;280:7131–7134. doi: 10.1074/jbc.M413038200. [DOI] [PubMed] [Google Scholar]
- Bartlam M, Yamamoto T. The structural basis for deadenylation by the CCR4-NOT complex. Protein Cell. 2010;1:443–452. doi: 10.1007/s13238-010-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SM, Campbell LT, Lodge JK. Cryptococcus neoformans, a fungus under stress. Current opinion in microbiology. 2007;10:320–325. doi: 10.1016/j.mib.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell DA, Kornberg RD. Complete, 12-subunit RNA polymerase II at 4.1-A resolution: implications for the initiation of transcription. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:6969–6973. doi: 10.1073/pnas.1130601100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabrier-Rosello Y, Gerik KJ, Koselny K, Didone L, Lodge JK, Krysan DJ. Cryptococcus neoformans phosphoinositide-dependent kinase 1 (PDK1) ortholog is required for stress tolerance and survival in murine phagocytes. Eukaryotic cell. 2012 doi: 10.1128/EC.00235-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan N, Inglis D, Roman E, Pla J, Li D, Calera JA, Calderone R. Candida albicans response regulator gene SSK1 regulates a subset of genes whose functions are associated with cell wall biosynthesis and adaptation to oxidative stress. Eukaryotic cell. 2003;2:1018–1024. doi: 10.1128/EC.2.5.1018-1024.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Shyu AB. Mechanisms of deadenylation-dependent decay. Wiley interdisciplinary reviews RNA. 2011;2:167–183. doi: 10.1002/wrna.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowart LA, Gandy JL, Tholanikunnel B, Hannun YA. Sphingolipids mediate formation of mRNA processing bodies during the heat-stress response of Saccharomyces cerevisiae. Biochem J. 2010;431:31–38. doi: 10.1042/BJ20100307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doidge R, Mittal S, Aslam A, Winkler GS. Deadenylation of cytoplasmic mRNA by the mammalian Ccr4-Not complex. Biochemical Society transactions. 2012;40:896–901. doi: 10.1042/BST20120074. [DOI] [PubMed] [Google Scholar]
- Dori-Bachash M, Shema E, Tirosh I. Coupled evolution of transcription and mRNA degradation. PLoS biology. 2011;9:e1001106. doi: 10.1371/journal.pbio.1001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farago M, Nahari T, Hammel C, Cole CN, Choder M. Rpb4p, a subunit of RNA polymerase II, mediates mRNA export during stress. Molecular biology of the cell. 2003;14:2744–2755. doi: 10.1091/mbc.E02-11-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerik KJ, Bhimireddy SR, Ryerse JS, Specht CA, Lodge JK. PKC1 is essential for protection against both oxidative and nitrosative stresses, cell integrity, and normal manifestation of virulence factors in the pathogenic fungus Cryptococcus neoformans. Eukaryotic cell. 2008;7:1685–1698. doi: 10.1128/EC.00146-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstrohm AC, Seay DJ, Hook BA, Wickens M. PUF protein-mediated deadenylation is catalyzed by Ccr4p. The Journal of biological chemistry. 2007;282:109–114. doi: 10.1074/jbc.M609413200. [DOI] [PubMed] [Google Scholar]
- Goler-Baron V, Selitrennik M, Barkai O, Haimovich G, Lotan R, Choder M. Transcription in the nucleus and mRNA decay in the cytoplasm are coupled processes. Genes & development. 2008;22:2022–2027. doi: 10.1101/gad.473608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel VE, Wool NK, Ayad D, Downey KM, Wilson CF, Larsen P, Djordjevic JT, Panepinto JC. Ccr4 promotes resolution of the endoplasmic reticulum stress response during host temperature adaptation in Cryptococcus neoformans. Eukaryotic cell. 2011;10:895–901. doi: 10.1128/EC.00006-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herruer MH, Mager WH, Raue HA, Vreken P, Wilms E, Planta RJ. Mild temperature shock affects transcription of yeast ribosomal protein genes as well as the stability of their mRNAs. Nucleic acids research. 1988;16:7917–7929. doi: 10.1093/nar/16.16.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heung LJ, Luberto C, Plowden A, Hannun YA, Del Poeta M. The sphingolipid pathway regulates Pkc1 through the formation of diacylglycerol in Cryptococcus neoformans. The Journal of biological chemistry. 2004;279:21144–21153. doi: 10.1074/jbc.M312995200. [DOI] [PubMed] [Google Scholar]
- Inagaki M, Schmelzle T, Yamaguchi K, Irie K, Hall MN, Matsumoto K. PDK1 homologs activate the Pkc1-mitogen-activated protein kinase pathway in yeast. Molecular and cellular biology. 1999;19:8344–8352. doi: 10.1128/mcb.19.12.8344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozubowski L, Aboobakar EF, Cardenas ME, Heitman J. Calcineurin colocalizes with P-bodies and stress granules during thermal stress in Cryptococcus neoformans. Eukaryotic cell. 2011;10:1396–1402. doi: 10.1128/EC.05087-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus PR, Boily MJ, Giles SS, Stajich JE, Allen A, Cox GM, Dietrich FS, Perfect JR, Heitman J. Identification of Cryptococcus neoformans temperature-regulated genes with a genomic-DNA microarray. Eukaryotic cell. 2004;3:1249–1260. doi: 10.1128/EC.3.5.1249-1260.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Khanal Lamichhane A, Garraffo HM, Kwon-Chung KJ, Chang YC. Involvement of PDK1, PKC and TOR signalling pathways in basal fluconazole tolerance in Cryptococcus neoformans. Molecular microbiology. 2012;84:130–146. doi: 10.1111/j.1365-2958.2012.08016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Zhang X, Lester RL, Dickson RC. The sphingoid long chain base phytosphingosine activates AGC-type protein kinases in Saccharomyces cerevisiae including Ypk1, Ypk2, and Sch9. The Journal of biological chemistry. 2005;280:22679–22687. doi: 10.1074/jbc.M502972200. [DOI] [PubMed] [Google Scholar]
- Liu OW, Chun CD, Chow ED, Chen C, Madhani HD, Noble SM. Systematic genetic analysis of virulence in the human fungal pathogen Cryptococcus neoformans. Cell. 2008;135:174–188. doi: 10.1016/j.cell.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan R, V, Bar-On G, Harel-Sharvit L, Duek L, Melamed D, Choder M. The RNA polymerase II subunit Rpb4p mediates decay of a specific class of mRNAs. Genes & development. 2005;19:3004–3016. doi: 10.1101/gad.353205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan R, Goler-Baron V, Duek L, Haimovich G, Choder M. The Rpb7p subunit of yeast RNA polymerase II plays roles in the two major cytoplasmic mRNA decay mechanisms. J Cell Biol. 2007;178:1133–1143. doi: 10.1083/jcb.200701165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luberto C, Toffaletti DL, Wills EA, Tucker SC, Casadevall A, Perfect JR, Hannun YA, Del Poeta M. Roles for inositol-phosphoryl ceramide synthase 1 (IPC1) in pathogenesis of C. neoformans. Genes & development. 2001;15:201–212. doi: 10.1101/gad.856001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G, Costanzo M, Boone C, Dickson RC. Nutrients and the Pkh1/2 and Pkc1 protein kinases control mRNA decay and P-body assembly in yeast. The Journal of biological chemistry. 2011;286:8759–8770. doi: 10.1074/jbc.M110.196030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G, Gruhler A, Liu Y, Jensen ON, Dickson RC. The sphingolipid long-chain base-Pkh1/2-Ypk1/2 signaling pathway regulates eisosome assembly and turnover. The Journal of biological chemistry. 2008;283:10433–10444. doi: 10.1074/jbc.M709972200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Ko YJ, Kim GB, Jung KW, Floyd A, Heitman J, Bahn YS. Comparative transcriptome analysis reveals novel roles of the Ras and cyclic AMP signaling pathways in environmental stress response and antifungal drug sensitivity in Cryptococcus neoformans. Eukaryotic cell. 2010;9:360–378. doi: 10.1128/EC.00309-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCluskey K, Wiest A, Plamann M. The Fungal Genetics Stock Center: a repository for 50 years of fungal genetics research. J Biosci. 2010;35:119–126. doi: 10.1007/s12038-010-0014-6. [DOI] [PubMed] [Google Scholar]
- Meka H, Werner F, Cordell SC, Onesti S, Brick P. Crystal structure and RNA binding of the Rpb4/Rpb7 subunits of human RNA polymerase II. Nucleic acids research. 2005;33:6435–6444. doi: 10.1093/nar/gki945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munchel SE, Shultzaberger RK, Takizawa N, Weis K. Dynamic profiling of mRNA turnover reveals gene-specific and system-wide regulation of mRNA decay. Molecular biology of the cell. 2011;22:2787–2795. doi: 10.1091/mbc.E11-01-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EL, Schoenberg DR. Assays for determining poly(A) tail length and the polarity of mRNA decay in mammalian cells. Methods Enzymol. 2008;448:483–504. doi: 10.1016/S0076-6879(08)02624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odom A, Muir S, Lim E, Toffaletti DL, Perfect J, Heitman J. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 1997;16:2576–2589. doi: 10.1093/emboj/16.10.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlicky SM, Tran PT, Sayre MH, Edwards AM. Dissociable Rpb4-Rpb7 subassembly of rna polymerase II binds to single-strand nucleic acid and mediates a post-recruitment step in transcription initiation. The Journal of biological chemistry. 2001;276:10097–10102. doi: 10.1074/jbc.M003165200. [DOI] [PubMed] [Google Scholar]
- Paldanius PM, Ivaska KK, Hovi P, Andersson S, Vaananen HK, Kajantie E, Makitie O. The effect of oral glucose tolerance test on serum osteocalcin and bone turnover markers in young adults. Calcified tissue international. 2012;90:90–95. doi: 10.1007/s00223-011-9551-8. [DOI] [PubMed] [Google Scholar]
- Panepinto J, Liu L, Ramos J, Zhu X, Valyi-Nagy T, Eksi S, Fu J, Jaffe HA, Wickes B, Williamson PR. The DEAD-box RNA helicase Vad1 regulates multiple virulence-associated genes in Cryptococcus neoformans. J Clin Invest. 2005;115:632–641. doi: 10.1172/JCI200523048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panepinto JC, Komperda KW, Hacham M, Shin S, Liu X, Williamson PR. Binding of serum mannan binding lectin to a cell integrity-defective Cryptococcus neoformans ccr4Delta mutant. Infection and immunity. 2007;75:4769–4779. doi: 10.1128/IAI.00536-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- Parker R, Sheth U. P bodies and the control of mRNA translation and degradation. Mol Cell. 2007;25:635–646. doi: 10.1016/j.molcel.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Perfect JR. Cryptococcus neoformans: the yeast that likes it hot. FEMS yeast research. 2006;6:463–468. doi: 10.1111/j.1567-1364.2006.00051.x. [DOI] [PubMed] [Google Scholar]
- Rendl LM, Bieman MA, Smibert CA. S. cerevisiae Vts1p induces deadenylation-dependent transcript degradation and interacts with the Ccr4p-Pop2p-Not deadenylase complex. RNA. 2008;14:1328–1336. doi: 10.1261/rna.955508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittershaus PC, Kechichian TB, Allegood JC, Merrill AH, Jr, Hennig M, Luberto C, Del Poeta M. Glucosylceramide synthase is an essential regulator of pathogenicity of Cryptococcus neoformans. J Clin Invest. 2006;116:1651–1659. doi: 10.1172/JCI27890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelants FM, Torrance PD, Thorner J. Differential roles of PDK1- and PDK2-phosphorylation sites in the yeast AGC kinases Ypk1, Pkc1 and Sch9. Microbiology. 2004;150:3289–3304. doi: 10.1099/mic.0.27286-0. [DOI] [PubMed] [Google Scholar]
- Runner VM, Podolny V, Buratowski S. The Rpb4 subunit of RNA polymerase II contributes to cotranscriptional recruitment of 3′ processing factors. Molecular and cellular biology. 2008;28:1883–1891. doi: 10.1128/MCB.01714-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano R, Martin H, Casamayor A, Arino J. Signaling alkaline pH stress in the yeast Saccharomyces cerevisiae through the Wsc1 cell surface sensor and the Slt2 MAPK pathway. The Journal of biological chemistry. 2006;281:39785–39795. doi: 10.1074/jbc.M604497200. [DOI] [PubMed] [Google Scholar]
- Shalem O, Groisman B, Choder M, Dahan O, Pilpel Y. Transcriptome kinetics is governed by a genome-wide coupling of mRNA production and degradation: a role for RNA Pol II. PLoS genetics. 2011;7:e1002273. doi: 10.1371/journal.pgen.1002273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth U, Parker R. Decapping and decay of messenger RNA occur in cytoplasmic processing bodies. Science. 2003;300:805–808. doi: 10.1126/science.1082320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Miao Y, Yamane Y, Zhang C, Shokat KM, Takematsu H, Kozutsumi Y, Drubin DG. Orm protein phosphoregulation mediates transient sphingolipid biosynthesis response to heat stress via the Pkh-Ypk and Cdc55-PP2A pathways. Molecular biology of the cell. 2012;23:2388–2398. doi: 10.1091/mbc.E12-03-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toffaletti DL, Rude TH, Johnston SA, Durack DT, Perfect JR. Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. J Bacteriol. 1993;175:1405–1411. doi: 10.1128/jb.175.5.1405-1411.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederhold K, Passmore LA. Cytoplasmic deadenylation: regulation of mRNA fate. Biochemical Society transactions. 2010;38:1531–1536. doi: 10.1042/BST0381531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.