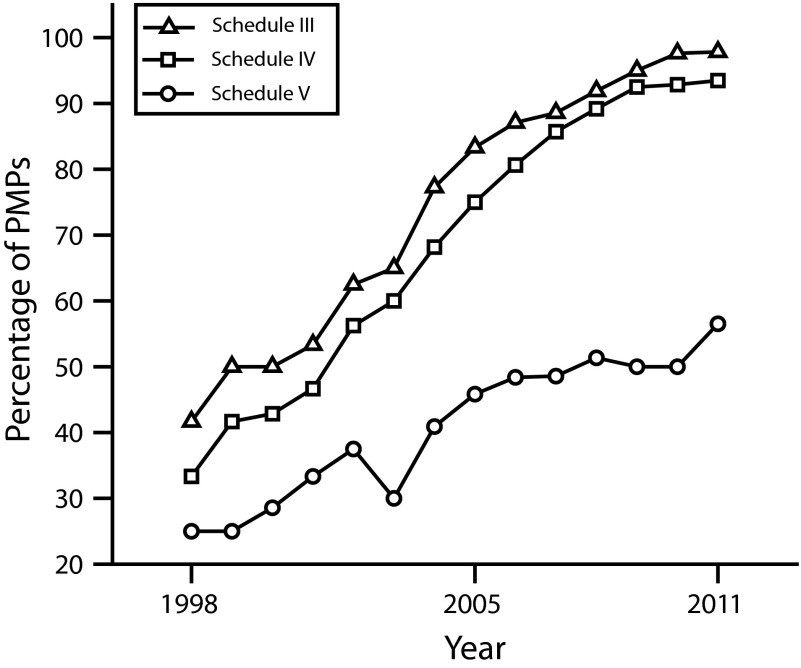
FIGURE 2—
Requirements for medicines in selected federal drug schedules to be included in state prescription monitoring programs: United States, 1998–2011.
Note. PMP = prescription monitoring program. All prescription monitoring programs required data on Schedule II medicines to be submitted during the entire study period. By 2011 nearly all states also required Schedules III and IV, but only approximately half required reporting of Schedule V medications.