Of the ∼22,000 protein-coding genes in the human genome, an estimated 8,000 are druggable (1). The current drug market targets no more than 450 unique human proteins (1) and many contain antihypertensive, antineoplastic, or anti-inflammatory effects. Receptors, enzymes, and transporter proteins comprise most of the targets. The following protein families are overrepresented: rhodopsin-like G protein-coupled receptors, voltage-gated ion channels, and ligand-gated ion channels (1). Their membrane localization, diverse tissue distribution, and the critical roles played by ion channels in human physiology makes these proteins attractive targets for drug discovery (2). Although worldwide sales of ion channel-targeted drugs are over US$10 billion, ion channels remain significantly underexploited as therapeutic targets (3).
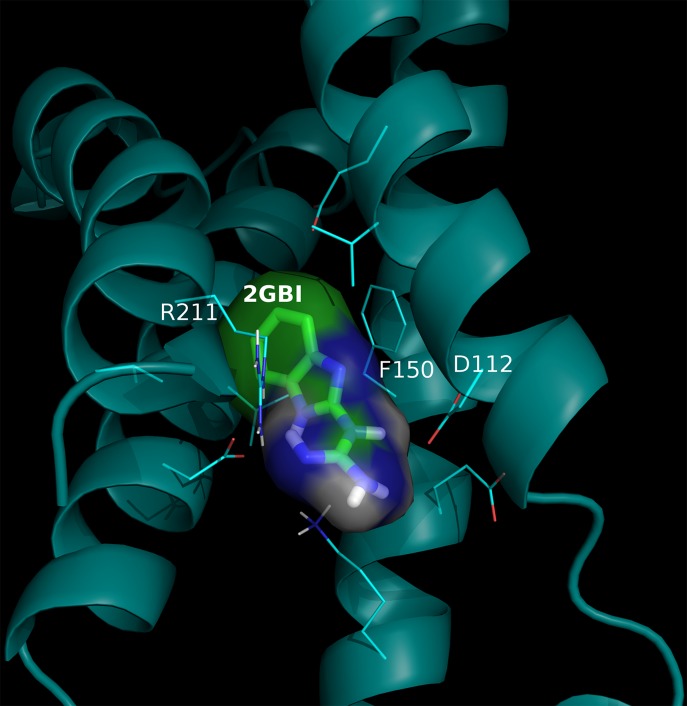
In PNAS, Hong et al. (4) identify residues aspartate 112, phenylalanine 150, serine 181, and arginine 211 from the Hv1 proton channel as being responsible for the binding guanidine derivatives, such as 2-guanidinobenzimidazole (2GBI). A representation of this complex interaction between 2GBI and the human Hv1 channel is shown in Fig. 1.
Fig. 1.
A probable interaction complex between 2GBI and the human Hv1 channel. 2GBI is docked against a homology model of the Hv1 channel based on the 4G7V_S template. 2GBI is represented by sticks with green carbon atoms and the Hv1 channel is represented by a cyan cartoon, with side chains of residues interacting with 2GBI represented as lines. From them, residues identified experimentally by Hong et al. (4) are labeled. This model is approximated and does not have further claims.
Hong et al. (4) characterize the binding affinity of 18 2GBI derivates on eight mutants of the human Hv1 channel through the application of a mutant cycle analysis (5). The authors also provide an improved derivate, the ClGBI, which can lead to the development of a therapeutic drug that is still an orphan target: the proton channel.
The Hv1 channel is involved in many physiological processes and is expressed in diverse tissues (6). One of its widely studied roles—one of particular importance to this study—is the coupling with the NADPH oxidase enzyme (NOX2) during respiratory bursts in phagocytes, thus compensating for pH and charge (6, 7). Reduction of neutrophil reactive oxygen species (ROS) generation by Hv1 inhibition is relevant for various inflammatory pathologies and different infectious diseases with a significant inflammatory component (7). Hv1 inhibition in eosinophil should reduce histamine secretion, which can be used in the treatment of allergies (7). Osteoclasts require NOX-dependent ROS generation for bone resorption (8), which makes the Hv1 channel an interesting target for the treatment of osteoporosis. Additional evidence (9) suggests that Hv1 inhibition could be beneficial for the treatment of strokes and perhaps other neurodegenerative processes (7). The Hv1 channel is important for keeping a mildly acid pH in the airway surface liquid and for facilitating ROS generation by dual oxidase 1 NADPH oxidases. Deregulation of such processes can be involved in diseases, such as asthma, cystic fibrosis, and chronic obstructive lung disease (7). Hv1 inhibition represents a possible therapeutic approach for certain lymphoid tumors with hyper-reactive B-cell receptor signaling (7). Hv1 expression remains associated with invasive and metastatic phenotypes of different tumors, such as gliomas (10), and those found in breast (11) and colorectal cancer (12). Hv1 expression is also considered to be crucial for male fertility (13), in that Hv1 activation can help treat male infertility and its inactivation could be used as a contraception method.
Fortunately, no severe malformations or diseases have been observed in Hv1-knockout mice (14). Apparently, a healthy organism can successfully compensate for the lack of proton channels, which increases the value of Hv1 channels as a potential drug target: pathological processes that highly depend on the activity of Hv1 channels can be attacked with minimal effects on tissues with normal physiologic behavior.
Besides the guanidine derivatives described by Hong et al. (4), just two other types of molecules are identified as Hv1 inhibitors: Zn2+ (and, to a lesser degree, other polyvalent cations) and Hanatoxin (a tarantula venom) (15). Although inhibition by Zn2+ represented the gold standard used to identify putative proton currents (7), its use as an inhibitory agent against Hv1 is limited because of Zn2+ involvement in more than 300 different cellular process, which can potentially be altered. Hanatoxin is not specific for Hv1; it binds to the paddle motif, which is highly conserved among different voltage-gated ion channels (15).
The report by Hong et al. (4) expands the results from a previous Hong et al. report (16), which had described guanidine derivatives that bind the Hv1 channel from the intracellular side of the membrane and act as potential channel blockers. The most effective of these compounds, namely 2GBI, can access the core of Hv1’s voltage-sensing domain only when the channel is in the open conformation. 2GBI is too polar to permeate the cytoplasmic membrane, which thus prevents 2GBI from being considered a potential drug to inhibit Hv1, despite its usefulness as a pharmacological (16).
From the perspective of drug development, one the most relevant aspects of the paper by Hong et al. (4) is the finding that a simple modification of 2GBI results in a compound with the capacity to permeate the cellular membrane and access the intracellular side of the channel, so as to block the proton current with an increased apparent binding affinity. Despite its advantages over 2GBI, two main concerns remain about the potential of ClGBI being a putative lead compound for the development of inhibitory drugs against Hv1: first, the relatively low potency and the uncertainty of its fine specificity against Hv1; second, a concern over how ClGBI compares with existing small drugs targeting ion channels. The answer to the latter concern is: acceptably well. Many ion channel drugs and drug candidates retained relatively low potency, specifically within the range of 0.1–5 µM even after extensive optimization (2). This situation is possibly a reflection of the current level of the drug discovery process for ion channels, where many potential targets have not yet been cloned, making screening campaigns impossible, or when possible, resulting in the identification of poor quality leads (2). Something similar occurred during early stages of kinase drug discovery, in which just a small subset of targets received a large amount of attention and good drugs eventually appeared. Within the ion channel superfamily, the largest amount of attention remains devoted to certain transient receptor potential, Nav, and Cav channels, leading to more potent, selective, and drug-like compounds (2). Nevertheless, despite being an important characteristic for drugs, an overemphasis on potency to drive lead optimization often results in the identification of highly potent leads with poor drug-like properties (3). Moreover, in vitro potency provides no strong correlate with therapeutic dose (17). The perceived benefit of high in vitro potency may be negated by poorer ADMET (absorption, distribution, metabolism, and excretion-toxicity) properties (17). Whether ClGBI or some derivate represents a good lead or not is yet to be proven, but their relatively low potency does not rule them out. Thus, what is there to say about fine specificity? In general, all drugs are slightly promiscuous and the effects they elicit depend on their interaction with numerous proteins throughout the body, not just the target protein (1). The majority of ion channel modulators in clinical use today are relatively unselective (2). Pore domains tend to be the most conserved regions of protein across subfamilies, with pore blockers being the least selective (2). Broad selectivity is usually a negative feature, causing increased
Hong et al. characterize the binding affinity of 18 2GBI derivates on eight mutants of the human Hv1 channel through the application of a mutant cycle analysis.
toxicity and unwanted side effects. However, in some cases a broad binding profile may be beneficial for polypharmacology, where inactivation of multiple pathways involved in a particular disease may lead to better treatment outcomes (1). The results of the case of ClGBI (4) or similar compounds remain to be seen.
Apart from the fact that understanding how 2GBI and its derivatives interact with Hv1 is a relevant step toward obtaining an inhibitory drug for Hv1, the residues identified by Hong et al. (4), which are involved in the binding process of these compounds, shed light on critical structural aspects of the Hv1 channel. To our knowledge, the Hong et al. report is the first published finding of residue Asp-112 [proposed as the selectivity filter of Hv1 channels (18)] intracellular accessibility in the open conformation, contrary to its previously proposed localization in the extracellular vestibule (19–21). The fact that Arg-211 is also involved in the binding of 2GBI and derivatives supports the hypothesis that D112 and R211 are close enough to interact electrostatically (22, 23). This finding could potentially modify the proposed structural mechanism of how D112 acts as the selectivity filter (20).
Based on current information, the results reported by Hong et al. (4) are welcome, not only for providing improved pharmacological tools for functional studies of Hv1 channels and structural insights into the open channel conformation, but also for the development of potential leads for a future therapeutic drug targeting the proton channel.
Supplementary Material
Acknowledgments
This work was supported by Fondecyt 1120802 (to C.G.L.), Anillo ACT1104 (to C.G.L.), Redes Internacionales 130006 (to C.G.L.), and a Conicyt doctoral fellowship (to A.P.). Centro Interdisciplinario de Neurociencias de Valparaiso is a Millennium Institute supported by the Millennium Scientific Initiative.
Footnotes
The authors declare no conflict of interest.
See companion article on page 9971.
References
- 1.Lahti JL, Tang GW, Capriotti E, Liu T, Altman RB. Bioinformatics and variability in drug response: A protein structural perspective. J R Soc Interface. 2012;9(72):1409–1437. doi: 10.1098/rsif.2011.0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagal SK, et al. Ion channels as therapeutic targets: A drug discovery perspective. J Med Chem. 2013;56(3):593–624. doi: 10.1021/jm3011433. [DOI] [PubMed] [Google Scholar]
- 3.Wickenden A, Priest B, Erdemli G. Ion channel drug discovery: Challenges and future directions. Future Med Chem. 2012;4(5):661–679. doi: 10.4155/fmc.12.4. [DOI] [PubMed] [Google Scholar]
- 4.Hong L, Kim IH, Tombola F. Molecular determinants of Hv1 proton channel inhibition by guanidine derivatives. Proc Natl Acad Sci USA. 2014;111:9971–9976. doi: 10.1073/pnas.1324012111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hidalgo P, MacKinnon R. Revealing the architecture of a K+ channel pore through mutant cycles with a peptide inhibitor. Science. 1995;268(5208):307–310. doi: 10.1126/science.7716527. [DOI] [PubMed] [Google Scholar]
- 6.DeCoursey TE, Hosler J. Philosophy of voltage-gated proton channels. J R Soc Interface. 2014;11(92):20130799. doi: 10.1098/rsif.2013.0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seredenina T, Demaurex N, Krause KH. Voltage-gated proton channels as novel drug targets: From NADPH oxidase regulation to sperm biology. Antioxid Redox Signal. 2014 doi: 10.1089/ars.2013.5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sasaki H, et al. Receptor activator of nuclear factor-kappaB ligand-induced mouse osteoclast differentiation is associated with switching between NADPH oxidase homologues. Free Radic Biol Med. 2009;47(2):189–199. doi: 10.1016/j.freeradbiomed.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 9.Wu L-J, et al. The voltage-gated proton channel Hv1 enhances brain damage from ischemic stroke. Nat Neurosci. 2012;15(4):565–573. doi: 10.1038/nn.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Zhang S, Li SJ. Zn(2+) induces apoptosis in human highly metastatic SHG-44 glioma cells, through inhibiting activity of the voltage-gated proton channel Hv1. Biochem Biophys Res Commun. 2013;438(2):312–317. doi: 10.1016/j.bbrc.2013.07.067. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, et al. Specific expression of the human voltage-gated proton channel Hv1 in highly metastatic breast cancer cells, promotes tumor progression and metastasis. Biochem Biophys Res Commun. 2011;412(2):353–359. doi: 10.1016/j.bbrc.2011.07.102. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Wu X, Li Q, Zhang S, Li SJ. Human voltage-gated proton channel hv1: A new potential biomarker for diagnosis and prognosis of colorectal cancer. PLoS ONE. 2013;8(8):e70550. doi: 10.1371/journal.pone.0070550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Platts AE, et al. Success and failure in human spermatogenesis as revealed by teratozoospermic RNAs. Hum Mol Genet. 2007;16(7):763–773. doi: 10.1093/hmg/ddm012. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki M, et al. Autoimmune disorder phenotypes in Hvcn1-deficient mice. Biochem J. 2013;450(2):295–301. doi: 10.1042/BJ20121188. [DOI] [PubMed] [Google Scholar]
- 15.Alabi AA, Bahamonde MI, Jung HJ, Kim JI, Swartz KJ. Portability of paddle motif function and pharmacology in voltage sensors. Nature. 2007;450(7168):370–375. doi: 10.1038/nature06266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong L, Pathak MM, Kim IH, Ta D, Tombola F. Voltage-sensing domain of voltage-gated proton channel Hv1 shares mechanism of block with pore domains. Neuron. 2013;77(2):274–287. doi: 10.1016/j.neuron.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gleeson MP, Hersey A, Montanari D, Overington J. Probing the links between in vitro potency, ADMET and physicochemical parameters. Nat Rev Drug Discov. 2011;10(3):197–208. doi: 10.1038/nrd3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Musset B, et al. Aspartate 112 is the selectivity filter of the human voltage-gated proton channel. Nature. 2011;480(7376):273–277. doi: 10.1038/nature10557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramsey IS, et al. An aqueous H+ permeation pathway in the voltage-gated proton channel Hv1. Nat Struct Mol Biol. 2010;17(7):869–875. doi: 10.1038/nsmb.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kulleperuma K, et al. Construction and validation of a homology model of the human voltage-gated proton channel hHV1. J Gen Physiol. 2013;141(4):445–465. doi: 10.1085/jgp.201210856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chamberlin A, et al. Hydrophobic plug functions as a gate in voltage-gated proton channels. Proc Natl Acad Sci USA. 2014;111(2):E273–E282. doi: 10.1073/pnas.1318018111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berger TK, Isacoff EY. The pore of the voltage-gated proton channel. Neuron. 2011;72(6):991–1000. doi: 10.1016/j.neuron.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wood ML, et al. Water wires in atomistic models of the Hv1 proton channel. Biochim Biophys Acta. 2012;1818(2):286–293. doi: 10.1016/j.bbamem.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]