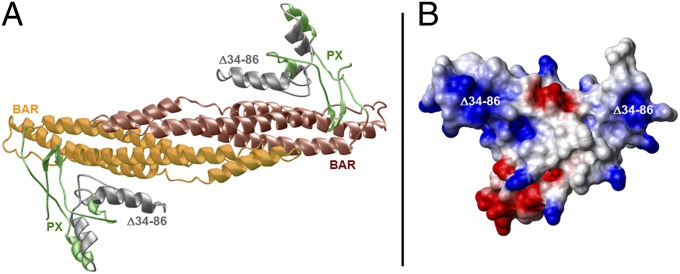
Fig. 3.
Structure of the PX–BAR module of human FAM65B. (A) Ribbon representation of the structural model of the predicted PX–BAR module, responsible for targeting and binding the protein to the cell membrane. The PX domain promotes membrane localization by binding to phospholipids. Together, the nexin PX and BAR domains both aid protein sorting and act as sensors of membrane curvature. The PX–BAR module is shown as a homodimer, with the N-terminal PX domain (residues 1–110) colored green and gray and the more C-terminal BAR domain (residues 111–300) colored yellow and brown. The region harboring the deletion of residues 34–86 (Δ34–86) is colored gray and maps to the core region of the PX domain, critical for its membrane localization. (B) Electrostatic surface potential map of the monomeric PX domain derived from the structural model of the PX–BAR dimeric module. Blue and red colors denote the density of positive and negative charges, respectively, whereas the apolar and polar surfaces are indicated by white and gray, respectively. The Δ34–86 deletion maps to the region within the PX domain harboring predominantly a net positive charge (colored blue).