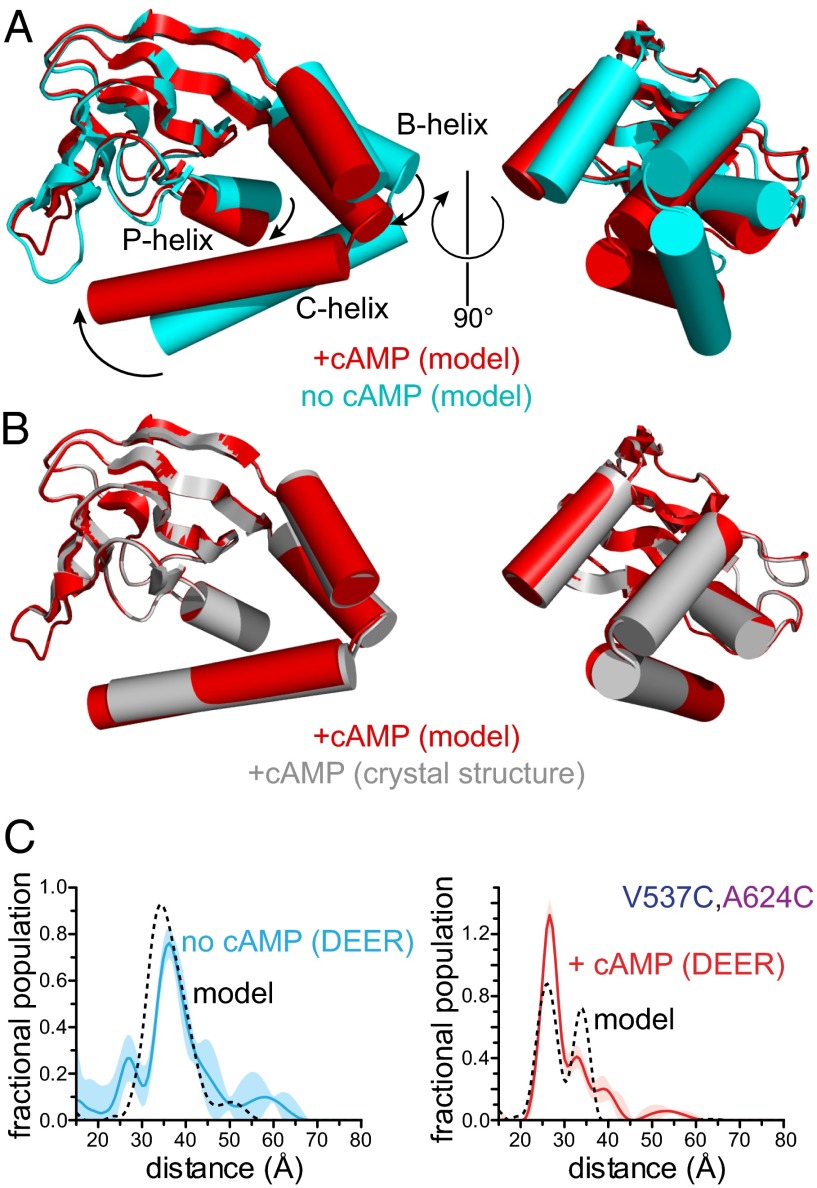
Fig. 4.
Conformational change in the HCN2 carboxy terminus induced by cAMP binding. (A) Elastic network models of the CNBD of HCN2 in the absence (cyan) and presence (red) of cAMP. Models were obtained using the HCN2cys-free crystal structure (PDB ID code 3ETQ) and experimental constraints from DEER. Structures were aligned at the β-rolls (residues 534–607). (B) Comparison between the modeled cAMP-bound structure (red) and the crystal structure of HCN2cys-free (gray) bound to cAMP (3ETQ). (C) Distance distributions obtained from DEER for V537C,A624C in the absence and presence of cAMP compared with predicted DEER traces (dashed lines) calculated from the modeled structures in A.